

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50257181 CHEMBL2325020
SMILES: CCN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1cscn1
InChI Key: InChIKey=FUEMKENJKYZACX-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257181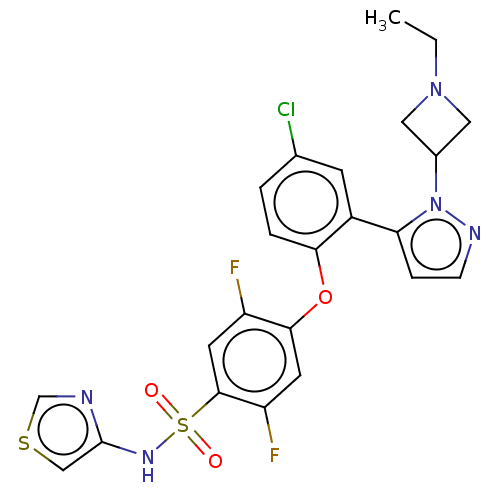 (CHEMBL2325020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50257181 (CHEMBL2325020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257181 (CHEMBL2325020) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NaV1.7 ion channel (unknown origin) | Bioorg Med Chem Lett 23: 261-3 (2013) Article DOI: 10.1016/j.bmcl.2012.10.102 BindingDB Entry DOI: 10.7270/Q2445QD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50257181 (CHEMBL2325020) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.5 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257181 (CHEMBL2325020) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||