

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50257541 (1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-benzo(d)imidazol-1-yl]piperidin-1-yl}propan-2-yl)-2-phenylcyclopropanecarboxamide (Inh_VU0359595)::CHEMBL524182::racemic trans-N-((S)-1-(4-(5-bromo-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidin-1-yl)propan-2-yl)-2-phenylcyclopropanecarboxamide
SMILES: C[C@@H](CN1CCC(CC1)n1c2ccc(Br)cc2[nH]c1=O)NC(=O)[C@@H]1C[C@H]1c1ccccc1
InChI Key: InChIKey=JSVNNLRZCJAYTQ-ORYQWCPZSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospholipase D (PLD) (Homo sapiens (Human)) | BDBM50257541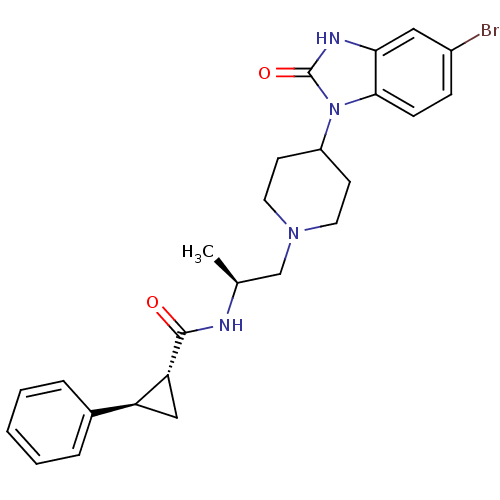 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Massachusetts Boston | Assay Description PLD enzymes (50 nM) were reconstituted with phospholipid vesicle substrates. All assays were performed at 37 C with agitation for 30 min. Reactions... | Chem Biol Drug Des 84: 270-81 (2014) Article DOI: 10.1111/cbdd.12319 BindingDB Entry DOI: 10.7270/Q2HT2N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (PLD2) (Homo sapiens (Human)) | BDBM50257541 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Massachusetts Boston | Assay Description PLD enzymes (50 nM) were reconstituted with phospholipid vesicle substrates. All assays were performed at 37 C with agitation for 30 min. Reactions... | Chem Biol Drug Des 84: 270-81 (2014) Article DOI: 10.1111/cbdd.12319 BindingDB Entry DOI: 10.7270/Q2HT2N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D (PLD_sp) (Streptomyces sp. PMF) | BDBM50257541 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Massachusetts Boston | Assay Description PLD enzymes (50 nM) were reconstituted with phospholipid vesicle substrates. All assays were performed at 37 C with agitation for 30 min. Reactions... | Chem Biol Drug Des 84: 270-81 (2014) Article DOI: 10.1111/cbdd.12319 BindingDB Entry DOI: 10.7270/Q2HT2N05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (PLD2) (Homo sapiens (Human)) | BDBM50257541 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of PLD2 (unknown origin) | Bioorg Med Chem Lett 28: 3670-3673 (2018) Article DOI: 10.1016/j.bmcl.2018.10.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D (PLD) (Homo sapiens (Human)) | BDBM50257541 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of human PLD1 in Calu1 cells | Bioorg Med Chem Lett 19: 1916-20 (2009) Article DOI: 10.1016/j.bmcl.2009.02.057 BindingDB Entry DOI: 10.7270/Q2VM4C5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D (PLD) (Homo sapiens (Human)) | BDBM50257541 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of PLD1 (unknown origin) | Bioorg Med Chem Lett 28: 3670-3673 (2018) Article DOI: 10.1016/j.bmcl.2018.10.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (PLD2) (Homo sapiens (Human)) | BDBM50257541 ((1R,2R)-N-([S]-1-{4-[5-bromo-2-oxo-2,3-dihydro-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of GFP-labelled human PLD2 HEK293 cells | Bioorg Med Chem Lett 19: 1916-20 (2009) Article DOI: 10.1016/j.bmcl.2009.02.057 BindingDB Entry DOI: 10.7270/Q2VM4C5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||