

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50262048 CHEMBL3134377
SMILES: Cc1ccc(cc1)-c1ncc(OC[C@@H]2CCNC2)cc1-c1ccc(cc1)C#N
InChI Key: InChIKey=IQVDLEXWAPYWDT-LJQANCHMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50262048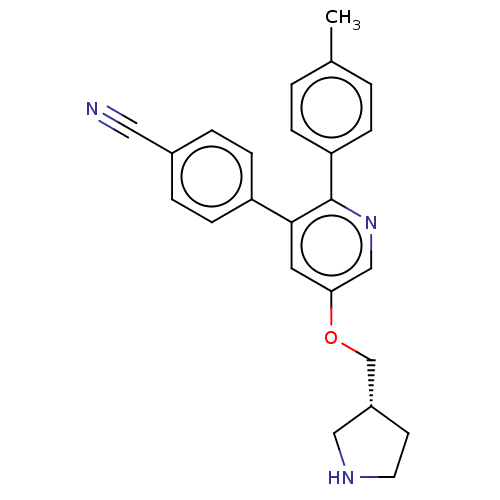 (CHEMBL3134377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal truncated LSD1 (151 to 852 residues) expressed in Escherichia coli using histone H3(1-21)K4(Me1) biotin pe... | Bioorg Med Chem Lett 27: 4755-4759 (2017) Article DOI: 10.1016/j.bmcl.2017.08.052 BindingDB Entry DOI: 10.7270/Q28918CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262048 (CHEMBL3134377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by Ionworks electrophysiology method | Bioorg Med Chem Lett 27: 4755-4759 (2017) Article DOI: 10.1016/j.bmcl.2017.08.052 BindingDB Entry DOI: 10.7270/Q28918CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50262048 (CHEMBL3134377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) | Bioorg Med Chem Lett 29: 103-106 (2019) Article DOI: 10.1016/j.bmcl.2018.11.001 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50262048 (CHEMBL3134377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of LSD1 in human THP1 cells assessed as induction of CD11b expression after 4 days by LIVE/DEAD violet staining based FACS analysis | Bioorg Med Chem Lett 29: 103-106 (2019) Article DOI: 10.1016/j.bmcl.2018.11.001 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50262048 (CHEMBL3134377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Unit, Cancer Research UK Manchester Institute, University of Manchester, Wilmslow Road, Manchester M20 4BX, UK. Electronic address: Daniel.mould@cruk.manchester.ac.uk. Curated by ChEMBL | Assay Description Binding affinity to LSD1 (unknown origin) by SPR analysis | Bioorg Med Chem Lett 27: 4755-4759 (2017) Article DOI: 10.1016/j.bmcl.2017.08.052 BindingDB Entry DOI: 10.7270/Q28918CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||