

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50262660 CHEMBL4065259
SMILES: CN1c2ccccc2C(N2CCSCC2)c2ccccc12
InChI Key: InChIKey=GYHIIYHXQOHZPU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50262660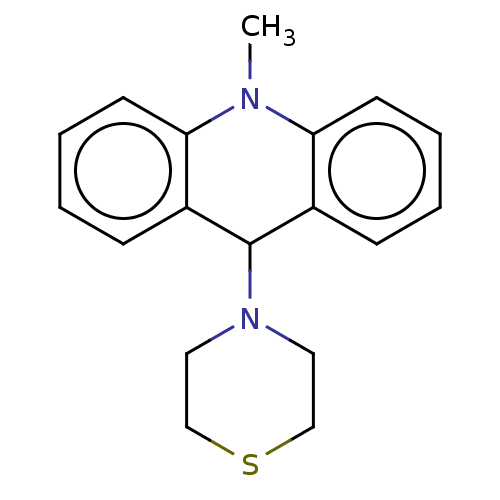 (CHEMBL4065259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262660 (CHEMBL4065259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262660 (CHEMBL4065259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262660 (CHEMBL4065259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylesterase (Sus scrofa) | BDBM50262660 (CHEMBL4065259) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||