

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50273709 2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylthio)octanoic acid::CHEMBL460616
SMILES: CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)C(O)=O
InChI Key: InChIKey=MLNIGXYHGQLWLD-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50273709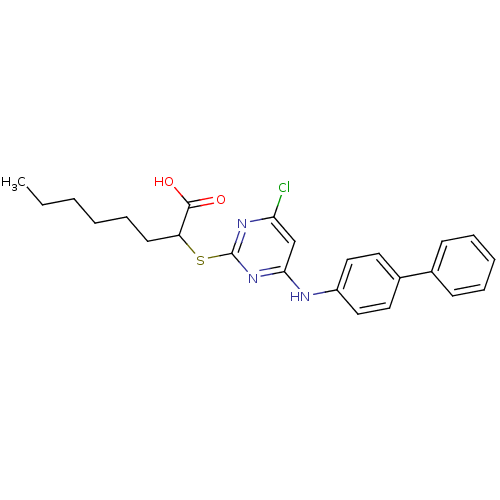 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 (unknown origin) | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli by cell-free assay | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-LO in PMNL cells | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX1 | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50273709 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of PGES1 in human A549 cell microsome assessed as PGE2 formation by cell-free assay | J Med Chem 51: 8068-76 (2008) Article DOI: 10.1021/jm801085s BindingDB Entry DOI: 10.7270/Q2639QN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||