 Found 7 hits for monomerid = 50277998
Found 7 hits for monomerid = 50277998 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase (cyclooxygenase)
(Ovis aries (Sheep)) | BDBM50277998
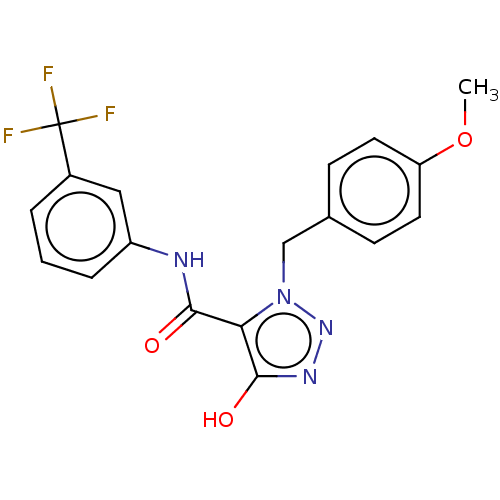
(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against retinoid X receptor using 5 nM of [3H]-9-cis-RA as a radioligand in baculovirus expressed receptor |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Aldo-keto-reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) using S-tetralol as substrate in presence of NADP+ by fluorimtery |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto-reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Thromboxane (TXA2) receptor antagonist activity using human platelet |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto-reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat... |
Eur J Med Chem 150: 930-945 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.040
BindingDB Entry DOI: 10.7270/Q2S46VMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50277998

(CHEMBL4174786)Show SMILES COc1ccc(Cn2nnc(O)c2C(=O)Nc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H15F3N4O3/c1-28-14-7-5-11(6-8-14)10-25-15(17(27)23-24-25)16(26)22-13-4-2-3-12(9-13)18(19,20)21/h2-9,27H,10H2,1H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C2 (unknown origin) using S-tetralol as substrate by by fluorimtery |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data