

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50279698 2-(4-Methyl-2-methylamino-thiazol-5-yl)-3-o-tolyl-3H-quinazolin-4-one::CHEMBL515276
SMILES: CNc1nc(C)c(s1)-c1nc2ccccc2c(=O)n1-c1ccccc1C
InChI Key: InChIKey=CNOZEZOCALUISD-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcription factor AP-1 (Homo sapiens (Human)) | BDBM50279698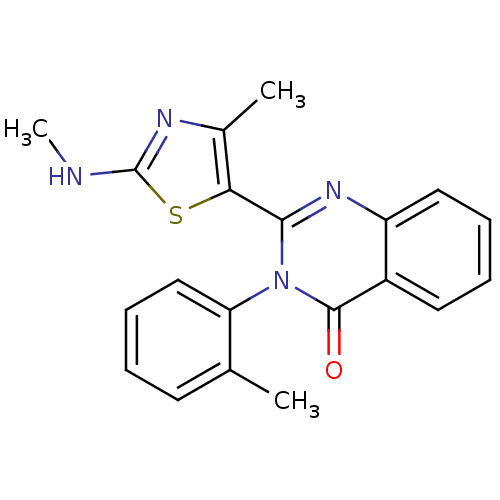 (2-(4-Methyl-2-methylamino-thiazol-5-yl)-3-o-tolyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
B.V. Patel Pharmaceutical Education and Research Development Centre Curated by ChEMBL | Assay Description Inhibition of AP1 (unknown origin) expressed in HEK293 cells assessed as inhibition of TNFalpha-induced transcriptional activation treated 24 hrs bef... | Eur J Med Chem 44: 2184-9 (2009) Article DOI: 10.1016/j.ejmech.2008.10.031 BindingDB Entry DOI: 10.7270/Q25X28SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor AP-1 (Homo sapiens (Human)) | BDBM50279698 (2-(4-Methyl-2-methylamino-thiazol-5-yl)-3-o-tolyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Inhibition of AP-1-mediated transcriptional activation in HEK293 cells by luciferase reporter gene assay | J Med Chem 57: 6930-48 (2014) Article DOI: 10.1021/jm5004733 BindingDB Entry DOI: 10.7270/Q2222WDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||