

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50287322 1-{3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-isopropyl-1H-indol-2-yl]-2,2-dimethyl-propyl}-1-hydroxy-urea::CHEMBL39189
SMILES: CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)CN(O)C(N)=O)c(SC(C)(C)C)c2c1
InChI Key: InChIKey=AIIZMHYSVIPRCK-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Lipoxygenase/5-lipoxygenase activating protein (Homo sapiens (Human)) | BDBM50287322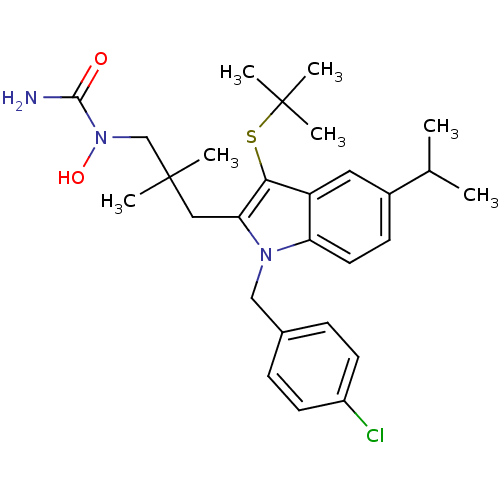 (1-{3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of calcium ionophore (A23187) stimulated LTB4 formation in human neutrophils | Bioorg Med Chem Lett 6: 1547-1552 (1996) Article DOI: 10.1016/S0960-894X(96)00271-5 BindingDB Entry DOI: 10.7270/Q2DF6R6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-Lipoxygenase/5-lipoxygenase activating protein (Homo sapiens (Human)) | BDBM50287322 (1-{3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of calcium ionophore (A23187) stimulated LTB4 formation in human neutrophils | Bioorg Med Chem Lett 6: 1547-1552 (1996) Article DOI: 10.1016/S0960-894X(96)00271-5 BindingDB Entry DOI: 10.7270/Q2DF6R6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50287322 (1-{3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of 5-lipoxygenase activity in a broken cell supernatant rat basophilic leukemia cells | Bioorg Med Chem Lett 6: 1547-1552 (1996) Article DOI: 10.1016/S0960-894X(96)00271-5 BindingDB Entry DOI: 10.7270/Q2DF6R6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||