

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50293171 (1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydroxy-1',2,4,6,8,12,14,19-octamethyl-23,25,26,27-tetraoxaspiro[pentacyclo[18.5.1.1^{11,15}.0^{1,22}.0^{16,21}]heptacosane-24,4'-piperidine]-6,16(21),19-triene-17,18-dione::CHEMBL508232
SMILES: C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC1(CCN(C)CC1)O4
InChI Key: InChIKey=ZFTJWLAKRPKMHQ-WALJHCINSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50293171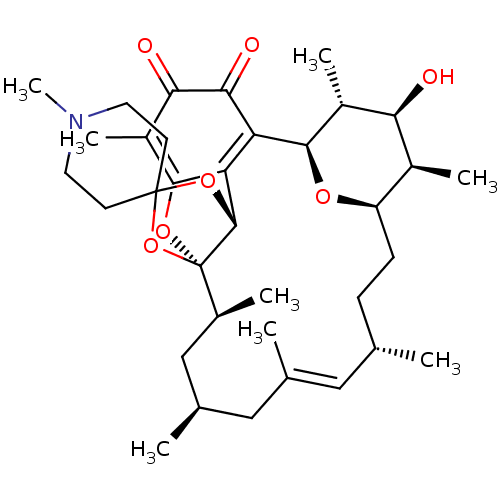 ((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.77E+5 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd Curated by ChEMBL | Assay Description Inhibition of interaction between GST-tagged Bcl-xl and Bak by surface plasmon resonance assay | Bioorg Med Chem Lett 18: 5771-3 (2009) Article DOI: 10.1016/j.bmcl.2008.09.071 BindingDB Entry DOI: 10.7270/Q2DF6R7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50293171 ((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd Curated by ChEMBL | Assay Description Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay | Bioorg Med Chem Lett 18: 5771-3 (2009) Article DOI: 10.1016/j.bmcl.2008.09.071 BindingDB Entry DOI: 10.7270/Q2DF6R7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50293171 ((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.99E+5 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd Curated by ChEMBL | Assay Description Inhibition of interaction between Bcl-xl and Bak by surface plasmon resonance assay | Bioorg Med Chem Lett 18: 5771-3 (2009) Article DOI: 10.1016/j.bmcl.2008.09.071 BindingDB Entry DOI: 10.7270/Q2DF6R7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||