

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50297400 (S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulfonyl-phenyl)-propyl]-benzamide::4-cyano-N-{(3S)-3-(4-fluorophenyl)-3-[4-(methylsulfonyl)phenyl]propyl}benzamide::CHEMBL556717
SMILES: CS(=O)(=O)c1ccc(cc1)[C@@H](CCNC(=O)c1ccc(cc1)C#N)c1ccc(F)cc1
InChI Key: InChIKey=OXJGQTIVCHEXQS-QHCPKHFHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400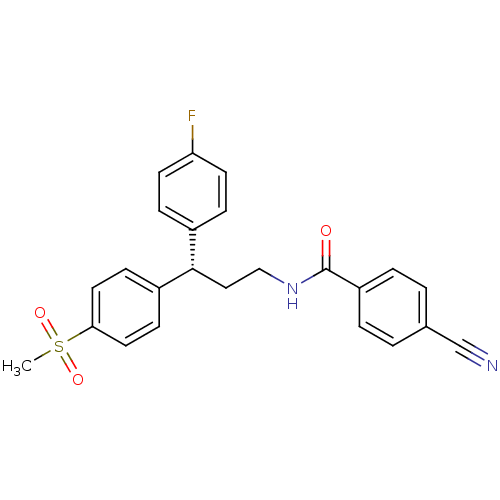 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epoxide hydrolase 2 (Rattus norvegicus) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a... | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assay | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2J2 (Homo sapiens (Human)) | BDBM50297400 ((S)-4-Cyano-N-[3-(4-fluorophenyl)-3-(4-methanesulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2J2 | J Med Chem 52: 5880-95 (2009) Article DOI: 10.1021/jm9005302 BindingDB Entry DOI: 10.7270/Q2BG2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||