

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50314498 CHEMBL1089299::N-((2S,3S)-2-(benzo[d][1,3]dioxol-5-yl)-3-((4S,5S)-5-((tert-butyldimethylsilyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-3-(methoxymethoxy)propyl)benzamide
SMILES: COCO[C@@H]([C@H](CNC(=O)c1ccccc1)c1ccc2OCOc2c1)[C@@H]1OC(C)(C)O[C@H]1CO[Si](C)(C)C(C)(C)C
InChI Key: InChIKey=PSRRUEPWXFJQOG-HBMTYJCASA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50314498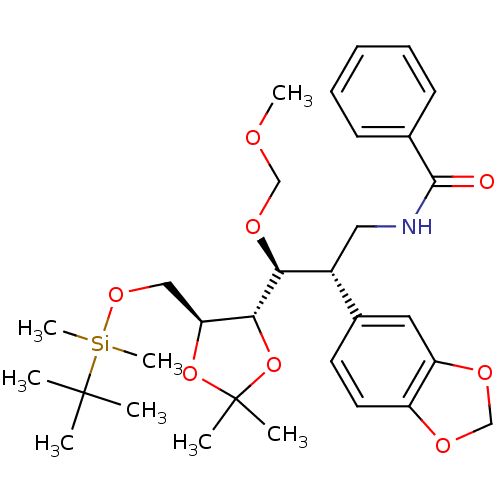 (CHEMBL1089299 | N-((2S,3S)-2-(benzo[d][1,3]dioxol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as biotransformation of 7-hydroxyquinoline to 7-benzyloxyquinoline measured every minute for 15 mins ... | Bioorg Med Chem Lett 20: 2335-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.157 BindingDB Entry DOI: 10.7270/Q21V5F4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||