

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50317179 2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]-beta-carboline-2-ium diiodide::CHEMBL1087458
SMILES: C[n+]1ccc2c(c1)n(CCCCCCCCCn1c3ccccc3c3cc[n+](C)cc13)c1ccccc21
InChI Key: InChIKey=XBLBEGLFURYCBO-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50317179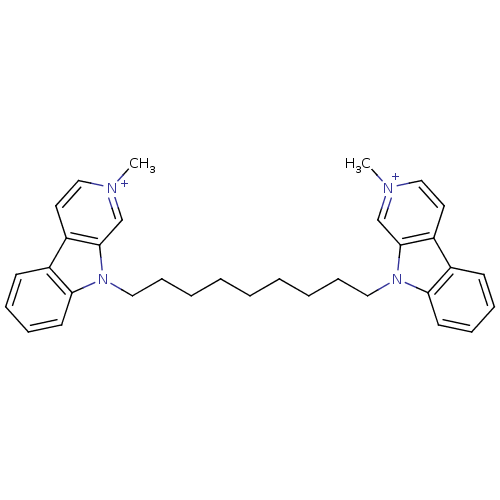 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 58: 6710-5 (2015) BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB | J Med Chem 58: 6710-5 (2015) BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BuChE) (Equus caballus (Horse)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.492 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NR1/NR2A (Homo sapiens (Human)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of dexamethasone-induced human NR1-1a/NR2A receptor-mediated excitotoxicity in (S)-glutamate/glycine-stimulated mouse L12-G10 cells assess... | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GluN1/GluN2B NMDA receptor (Homo sapiens (Human)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of dexamethasone-induced human NR1-1a/NR2B receptor-mediated excitotoxicity in (S)-glutamate/glycine-stimulated mouse L13-E6 cells assesse... | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BuChE) (Equus caballus (Horse)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.507 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||