

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50323714 CHEMBL1213206::CHEMBL1213210::N-(4-((Z)-(4-(2-(((2R,5S,8S,11S,14S,17S,23S,26S,29S,32S)-17-ethyl-14-((1R,2R,E)-1-hydroxy-2-methylhex-4-enyl)-5,8,23,29-tetraisobutyl-11,26-diisopropyl-4,7,10,13,19,22,28,32-octamethyl-3,6,9,12,15,18,21,24,27,30,33-undecaoxo-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2-yl)methoxy)acetamido)phenyl)diazenyl)phenyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide
SMILES: CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](COCC(=O)Nc2ccc(cc2)N=Nc2ccc(NC(=O)CCCC[C@@H]3SC[C@@H]4NC(=O)N[C@H]34)cc2)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C
InChI Key: InChIKey=DRYOHQIGNKGEAS-STSUPSDUSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50323714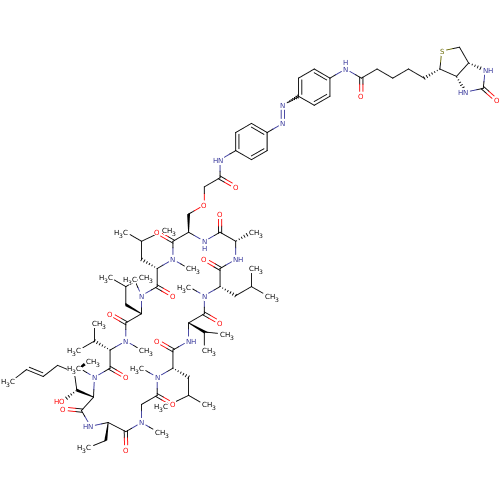 (CHEMBL1213206 | CHEMBL1213210 | N-(4-((Z)-(4-(2-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Binding affinity to cyclophilin 18 assessed as reduction in [33P]phosphatase activity of calcineurin by protease coupled assay | Nat Chem Biol 5: 724-6 (2009) Article DOI: 10.1038/nchembio.214 BindingDB Entry DOI: 10.7270/Q27D2VBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| calcineurin (Homo sapiens (Human)) | BDBM50323714 (CHEMBL1213206 | CHEMBL1213210 | N-(4-((Z)-(4-(2-((...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of calcineurin-mediated NFAT activation in human Jurkat cells by luciferase reporter gene assay | Nat Chem Biol 5: 724-6 (2009) Article DOI: 10.1038/nchembio.214 BindingDB Entry DOI: 10.7270/Q27D2VBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| calcineurin (Homo sapiens (Human)) | BDBM50323714 (CHEMBL1213206 | CHEMBL1213210 | N-(4-((Z)-(4-(2-((...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of calcineurin-mediated streptavidin-coupled NFAT activation in human Jurkat cells measured after 45 min of irradiation with 740 nm light ... | Nat Chem Biol 5: 724-6 (2009) Article DOI: 10.1038/nchembio.214 BindingDB Entry DOI: 10.7270/Q27D2VBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| calcineurin (Homo sapiens (Human)) | BDBM50323714 (CHEMBL1213206 | CHEMBL1213210 | N-(4-((Z)-(4-(2-((...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of calcineurin-mediated streptavidin-coupled NFAT activation in human Jurkat cells by luciferase reporter gene assay | Nat Chem Biol 5: 724-6 (2009) Article DOI: 10.1038/nchembio.214 BindingDB Entry DOI: 10.7270/Q27D2VBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| calcineurin (Homo sapiens (Human)) | BDBM50323714 (CHEMBL1213206 | CHEMBL1213210 | N-(4-((Z)-(4-(2-((...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding Curated by ChEMBL | Assay Description Inhibition of calcineurin-mediated NFAT activation in human Jurkat cells measured after 45 min of irradiation with 740 nm light by luciferase reporte... | Nat Chem Biol 5: 724-6 (2009) Article DOI: 10.1038/nchembio.214 BindingDB Entry DOI: 10.7270/Q27D2VBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||