

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50343137 CHEMBL1770313::Cyclomorusin::Cycolmorusin, 2
SMILES: [#6]\[#6](-[#6])=[#6]\[#6]-1-[#8]-c2cc(-[#8])ccc2-c2oc3c4-[#6]=[#6]C([#6])([#6])[#8]-c4cc(-[#8])c3c(=O)c12
InChI Key: InChIKey=GDQXJMLXEYSICD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50343137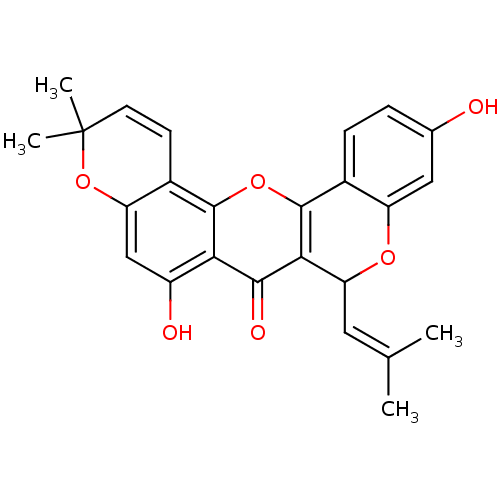 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 46.4 | -10.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50343137 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) | Citation and Details Article DOI: 10.1007/s00044-012-0353-y BindingDB Entry DOI: 10.7270/Q29Z97T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50343137 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.61E+5 | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50343137 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE-1 expressed in HEK293 cells assessed as inhibition of amyloid precursor protein cleavage into amyloid beta after... | Bioorg Med Chem Lett 21: 2945-8 (2011) Article DOI: 10.1016/j.bmcl.2011.03.060 BindingDB Entry DOI: 10.7270/Q2QR4XF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50343137 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE (unknown origin) | Citation and Details Article DOI: 10.1007/s00044-012-0353-y BindingDB Entry DOI: 10.7270/Q29Z97T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50343137 (CHEMBL1770313 | Cyclomorusin | Cycolmorusin, 2) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.16E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||