

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50355521 CHEMBL1910545
SMILES: Cc1ccc(Cn2cc(CN(C3CC3)C(=O)[C@H]3CNCC[C@]3(O)c3ccc(F)c(F)c3)c3c(F)cccc23)cn1
InChI Key: InChIKey=LVQKKHWEPPQIMP-XJFCNFDWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50355521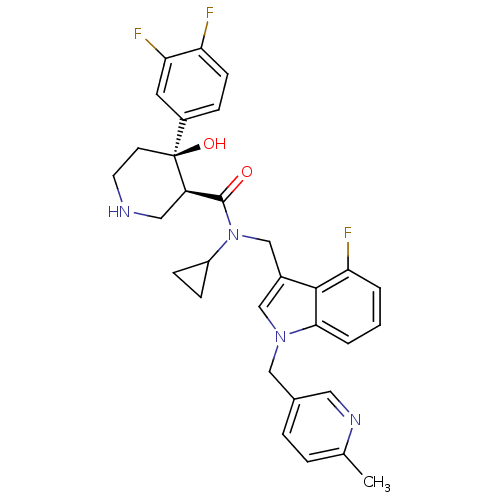 (CHEMBL1910545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355521 (CHEMBL1910545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as Q-FRET substrate after 3 hrs by fluorescence assay | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50355521 (CHEMBL1910545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in human plasma using QXL520-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-Lys-(5-FAM) as Q-FRET substrate after 1 hr by ... | Bioorg Med Chem Lett 21: 3976-81 (2011) Article DOI: 10.1016/j.bmcl.2011.05.014 BindingDB Entry DOI: 10.7270/Q21N81HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||