

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50366682 CHEMBL1627395
SMILES: CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O
InChI Key: InChIKey=VXKTZBKORWNMBL-OPMJLWCUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50366682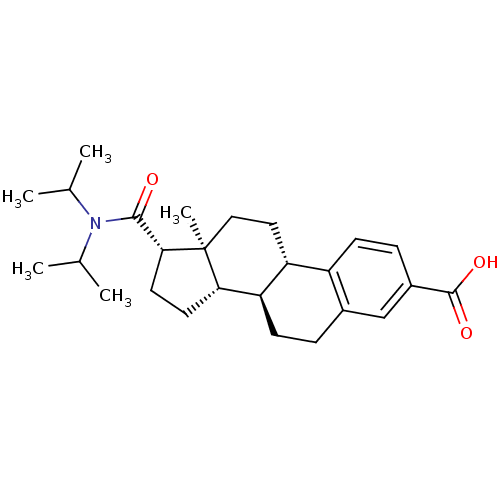 (CHEMBL1627395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase | Bioorg Med Chem Lett 1: 27-32 (1991) Article DOI: 10.1016/S0960-894X(01)81084-2 BindingDB Entry DOI: 10.7270/Q29P31J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Rattus norvegicus) | BDBM50366682 (CHEMBL1627395) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue | J Med Chem 33: 937-42 (1990) BindingDB Entry DOI: 10.7270/Q2F76DR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50366682 (CHEMBL1627395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||