

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50367063 CHEMBL4174199
SMILES: Cc1cc(cc2c1[nH]c(=O)c1ccccc21)C(O)(C(F)(F)F)C(F)(F)F
InChI Key: InChIKey=ZJIJGQSUYGWVGU-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50367063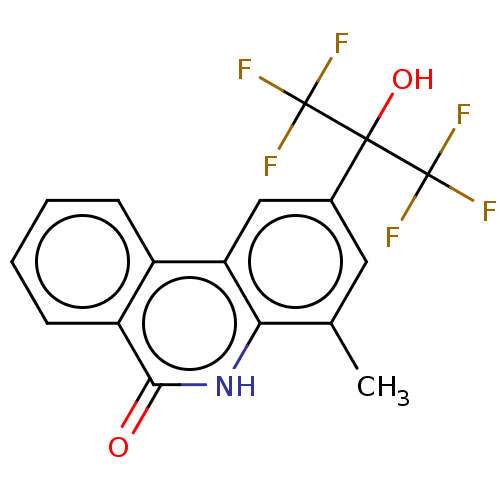 (CHEMBL4174199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human GAL4N-fused LXRalpha LBD expressed in HEK293 cells assessed as reduction in T0901317-induced transactivation activity af... | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB MMDB Reactome pathway UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human RORbeta1 expressed in HEK293 cells after 24 hrs by luciferase reporter gene assay | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity measured after 24 hrs | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human CMX-GRalpha expressed in HEK293 cells assessed as reduction in dexamethasone-induced transactivation activity after 24 h... | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human RORgamma1 expressed in HEK293 cells after 24 hrs by luciferase reporter gene assay | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human GAL4N-fused LXRbeta LBD expressed in HEK293 cells assessed as reduction in T0901317-induced transactivation activity aft... | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human CMX-AR expressed in HEK293 cells assessed as reduction in dihydrotestosterone-induced transactivation activity after 24 ... | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50367063 (CHEMBL4174199) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Displacement of [1,2,6,7-3H]progesterone from recombinant human GST-tagged PGR LBD (678 to 933 residues) expressed in baculovirus-infected insect cel... | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||