

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50400541 CHEMBL2204950
SMILES: COc1cccc(CNC(=O)N[C@@H](CC(C)C)C(=O)NO)c1
InChI Key: InChIKey=VRQZXSUCWGBJIY-ZDUSSCGKSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50400541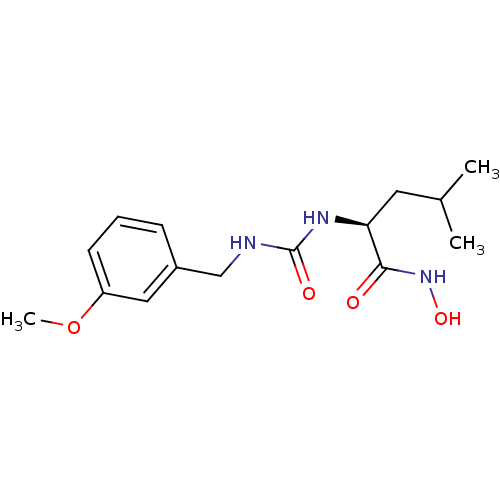 (CHEMBL2204950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APN in porcine kidney microsome assessed as inhibition of L-Leu-p-nitroanilide substrate hydrolysis incubated for 5 mins before substra... | ACS Med Chem Lett 3: 959-964 (2012) Article DOI: 10.1021/ml3000758 BindingDB Entry DOI: 10.7270/Q2QJ7JFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50400541 (CHEMBL2204950) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of APN in human ES2 cell surface assessed as inhibition of L-Leu-p-nitroanilide substrate hydrolysis incubated for 5 mins before substrate... | ACS Med Chem Lett 3: 959-964 (2012) Article DOI: 10.1021/ml3000758 BindingDB Entry DOI: 10.7270/Q2QJ7JFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50400541 (CHEMBL2204950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant MMP-2 using succinylated gelatin as substrate incubated for 10 mins before addition of substrate measured after 60 mins by ... | ACS Med Chem Lett 3: 959-964 (2012) Article DOI: 10.1021/ml3000758 BindingDB Entry DOI: 10.7270/Q2QJ7JFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Mus musculus) | BDBM50400541 (CHEMBL2204950) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse APN | ACS Med Chem Lett 3: 959-964 (2012) Article DOI: 10.1021/ml3000758 BindingDB Entry DOI: 10.7270/Q2QJ7JFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||