

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@]12CC[C@H]3[C@@H](CCc4ccccc34)[C@@H]1CC[C@@H]2O
InChI Key: InChIKey=MUENRDYXOADTOC-ZBRFXRBCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfotransferase (Sus scrofa) | BDBM50405375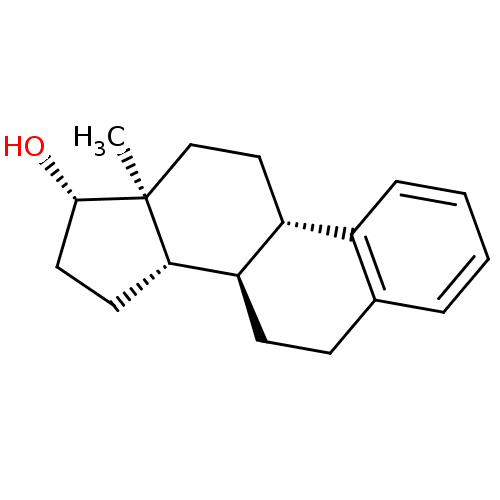 (CHEMBL194749) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine endometrial estrogen sulfotransferase | J Med Chem 29: 692-8 (1986) BindingDB Entry DOI: 10.7270/Q2M32WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50405375 (CHEMBL194749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881) | J Med Chem 48: 5666-74 (2005) Article DOI: 10.1021/jm050403f BindingDB Entry DOI: 10.7270/Q2TM7CBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||