

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(cc1)-c1c(Br)cn(Cc2ccc(F)cc2)c(=O)c1C(=O)NC1CCCCCC1
InChI Key: InChIKey=MJXAKHLEXLHOOP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50408217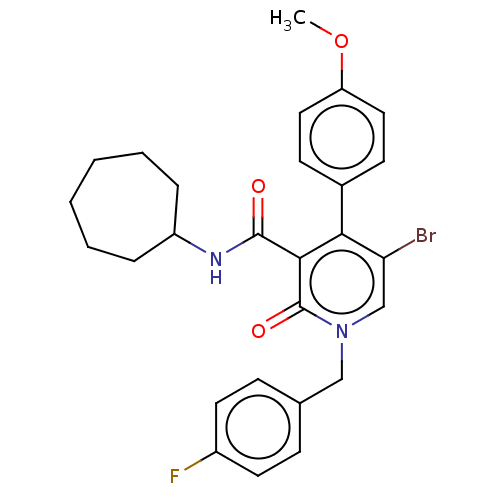 (CHEMBL4173284) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cell membranes after 90 mins | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50408217 (CHEMBL4173284) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cell membranes after 90 mins | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50408217 (CHEMBL4173284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Inhibition of MAGL in human U937 cells using 2-OG containing [glycerol-1,2,3-3H]AEA as substrate preincubated for 30 mins followed by substrate addit... | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoacylglycerol lipase ABHD6 (Homo sapiens (Human)) | BDBM50408217 (CHEMBL4173284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Inhibition of human ABHD6 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured afte... | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50408217 (CHEMBL4173284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Inhibition of FAAH in human U937 cells using AEA containing [ethanolamine-1-3H]AEA as substrate preincubated for 30 mins followed by substrate additi... | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidylserine lipase ABHD12 (Homo sapiens (Human)) | BDBM50408217 (CHEMBL4173284) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern Curated by ChEMBL | Assay Description Inhibition of human ABHD12 expressed in HEK293 cells using 2-OG as substrate preincubated for 30 mins followed by substrate addition and measured aft... | Eur J Med Chem 154: 155-171 (2018) Article DOI: 10.1016/j.ejmech.2018.05.019 BindingDB Entry DOI: 10.7270/Q24F1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||