

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50440736 CHEMBL2431088
SMILES: Cc1noc(N)c1-c1ccccc1
InChI Key: InChIKey=ZCTBUBMRXYVEHX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50440736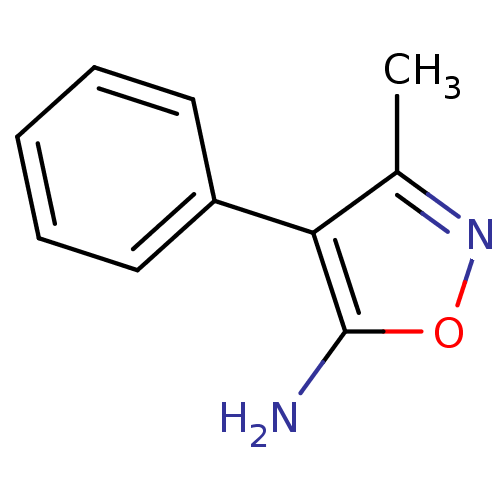 (CHEMBL2431088) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of His-FLAG-tagged BRD4 binding domain1 (unknown origin) binding to H4-TetraAc-biotin peptide after 20 mins by AlphaLISA | ACS Med Chem Lett 4: 835-40 (2013) Article DOI: 10.1021/ml4001485 BindingDB Entry DOI: 10.7270/Q2DV1MB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50440736 (CHEMBL2431088) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taros Chemicals GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibition of BRD4 bromodomain-1 (unknown origin) by TR-FRET assay | Eur J Med Chem 167: 76-95 (2019) Article DOI: 10.1016/j.ejmech.2019.01.084 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50440736 (CHEMBL2431088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO2 assessed as decrease in conversion of L-tryptophan to N-formylkynurenine preincubated for 5 mins followed by 0.2... | ACS Med Chem Lett 9: 417-421 (2018) Article DOI: 10.1021/acsmedchemlett.7b00427 BindingDB Entry DOI: 10.7270/Q2M0481G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50440736 (CHEMBL2431088) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of TDO2 in human SW48 cells assessed as decrease in conversion of tryptophan to N-formylkynurenine after 30 mins by fluorescence assay | ACS Med Chem Lett 9: 417-421 (2018) Article DOI: 10.1021/acsmedchemlett.7b00427 BindingDB Entry DOI: 10.7270/Q2M0481G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||