

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50441482 CHEMBL2436577
SMILES: Cc1cnc(cn1)C(=O)N1CCC2(CCN(C2)C(=O)Nc2ccc(OC(F)(F)F)cc2)CC1
InChI Key: InChIKey=RSLYDLGWAOOXOL-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50441482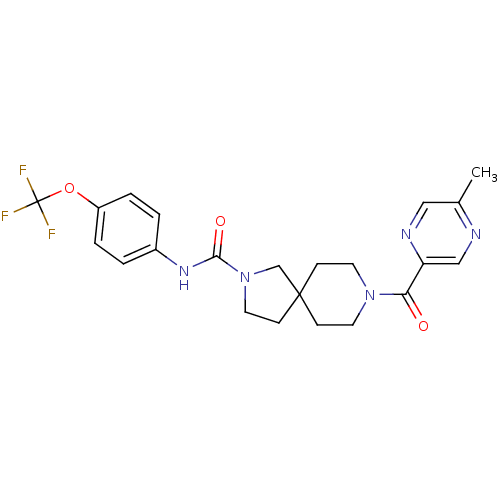 (CHEMBL2436577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of rat sEH expressed in Sf9 insect cells using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 2... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50441482 (CHEMBL2436577) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50441482 (CHEMBL2436577) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc. Curated by ChEMBL | Assay Description Inhibition of mouse sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by fluorescence... | Bioorg Med Chem Lett 23: 5975-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.054 BindingDB Entry DOI: 10.7270/Q2TT4SDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||