

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50441624 GLABRONE
SMILES: CC1(C)Oc2ccc(c(O)c2C=C1)-c1coc2cc(O)ccc2c1=O
InChI Key: InChIKey=COLMVFWKLOZOOP-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50441624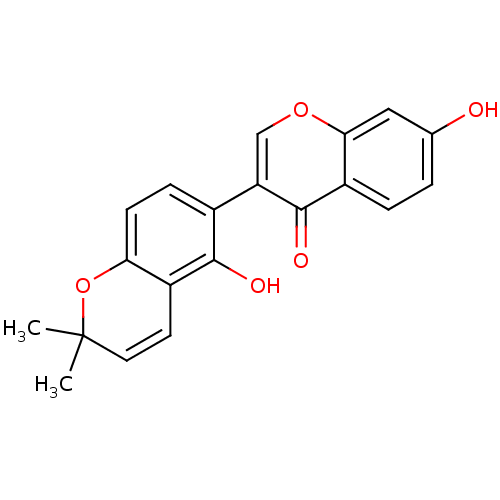 (GLABRONE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate | Bioorg Med Chem Lett 23: 5836-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.102 BindingDB Entry DOI: 10.7270/Q28W3FQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624 (GLABRONE) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus J/8178/09 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624 (GLABRONE) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Hong Kong/1/1968(H3N2)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624 (GLABRONE) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Puerto Rico/8/1934(H1N1)) neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50441624 (GLABRONE) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus H1N1 B/55/08 neuraminidase by chemiluminescence based assay | J Nat Prod 77: 563-70 (2014) Article DOI: 10.1021/np400817j BindingDB Entry DOI: 10.7270/Q23J3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||