

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@@]12CC=C([C@@H](C)CCO)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H]
InChI Key: InChIKey=TTWLNSHTBPTSHV-DZIGDOIQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50494961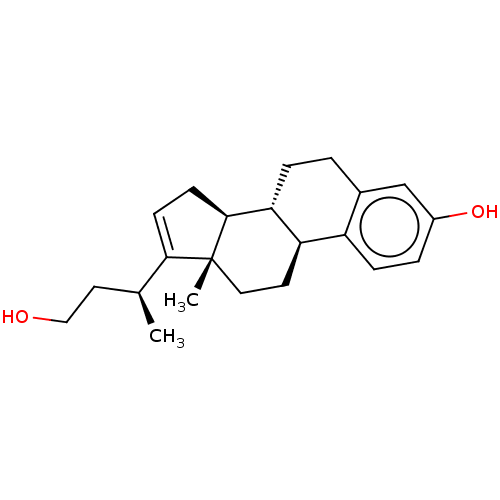 (CHEMBL3099428 | US10570077, Compound 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
Marquette University; Concordia University Inc. US Patent | Assay Description Twelve compounds from Schemes 1 and 2 were screened using fluorescence polarization, for their ability to bind ERα (Table 1). Only six compounds... | US Patent US10570077 (2020) BindingDB Entry DOI: 10.7270/Q2P271H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50494961 (CHEMBL3099428 | US10570077, Compound 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Displacement of FITC-estradiol from human ERalpha by fluorescence polarization assay | Bioorg Med Chem 22: 303-10 (2014) Article DOI: 10.1016/j.bmc.2013.11.024 BindingDB Entry DOI: 10.7270/Q2FF3WBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50494961 (CHEMBL3099428 | US10570077, Compound 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at ERbeta (unknown origin) assessed as inhibition of E2-induced receptor activation after 22 hrs by cell-based luciferase reporte... | Bioorg Med Chem 22: 303-10 (2014) Article DOI: 10.1016/j.bmc.2013.11.024 BindingDB Entry DOI: 10.7270/Q2FF3WBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50494961 (CHEMBL3099428 | US10570077, Compound 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at ERbeta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | Bioorg Med Chem 22: 303-10 (2014) Article DOI: 10.1016/j.bmc.2013.11.024 BindingDB Entry DOI: 10.7270/Q2FF3WBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||