

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50509326 CHEMBL4454696
SMILES: [H][C@]12[C@H](\C=C(/CO)C(=O)OC[C@]3(O)CC[C@H](C(C)C)[C@@H](\C=C(/CO)C(=O)O[C@]45COC(=O)[C@@]4([H])[C@H](\C=C(/CO)C(O)=O)[C@H](CC5)C(C)C)[C@@H]3C(O)=O)[C@H](CC[C@@]1(O)COC2=O)C(C)C
InChI Key: InChIKey=VUDGRKLUMAAASW-OGFCOVEESA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11A1 (Rattus norvegicus) | BDBM50509326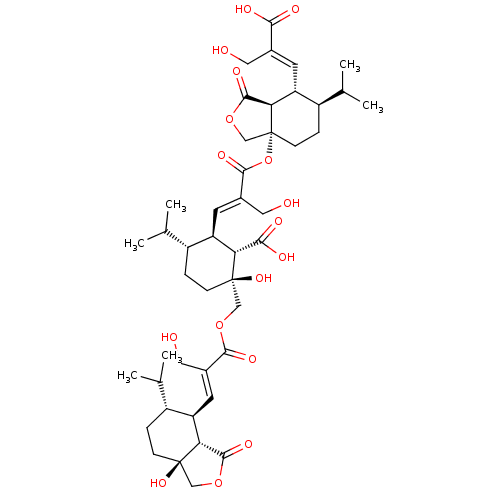 (CHEMBL4454696) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antibacterial activity against vancomycin-resistant Enterococcus faecalis AUS-RBWH-VRE-01 assessed as reduction in fungal growth incubated for 24 hrs... | J Nat Prod 82: 3165-3175 (2019) Article DOI: 10.1021/acs.jnatprod.9b00760 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||