

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50514056 CHEMBL4588547
SMILES: CCOc1ccc(Cc2nc3cc(ccc3n2CCC(C)C)C(=O)NCCNc2c3CCCCc3nc3ccccc23)cc1
InChI Key: InChIKey=UIKPRYUVBQFRSX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514056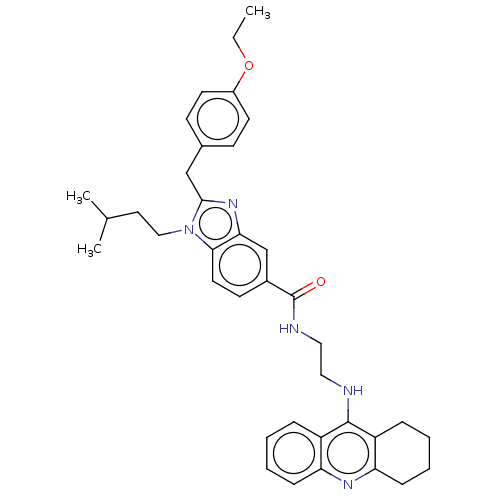 (CHEMBL4588547) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514056 (CHEMBL4588547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50514056 (CHEMBL4588547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||