

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCOC(=O)N1CCC2(CC1)C[C@H](NC(=O)c1cccc3cn[nH]c13)c1cc(Br)ccc1O2
InChI Key: InChIKey=XVMOVNVQGRDOCX-IBGZPJMESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514603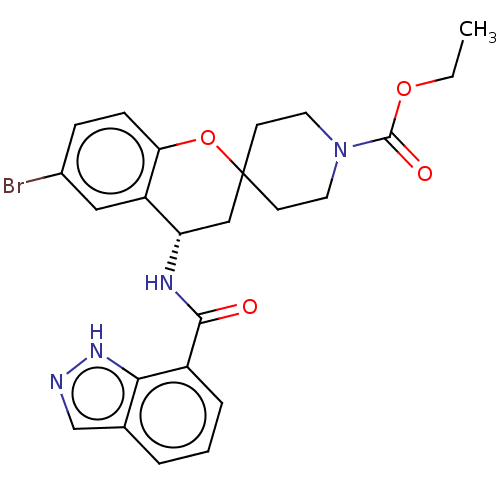 (CHEMBL4550298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant 6His/FLAG/Avi-tagged/Tev-fused IDO1 holoenzyme (unknown origin) expressed in Escherichia coli BL21 (DE3) using D-TRP as sub... | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514603 (CHEMBL4550298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant 6His/FLAG/Avi-tagged/Tev-fused IDO1 holoenzyme (unknown origin) expressed in Escherichia coli BL21 (DE3) using D-TRP as sub... | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514603 (CHEMBL4550298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50514603 (CHEMBL4550298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma/LPS stimulated human PBMC assessed as reduction in kynurenine production after 48 hrs by RapidFire MS assay | J Med Chem 63: 3552-3562 (2020) Article DOI: 10.1021/acs.jmedchem.9b01799 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||