

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50526302 CHEMBL4464826
SMILES: CCOc1cc(ccc1Nc1ncc2N(C)C(=O)[C@@H](CC)N(C3CCCC3)c2n1)C(=O)NC1CCN(C)CC1
InChI Key: InChIKey=DBBQRLYYNNWGPM-HSZRJFAPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526302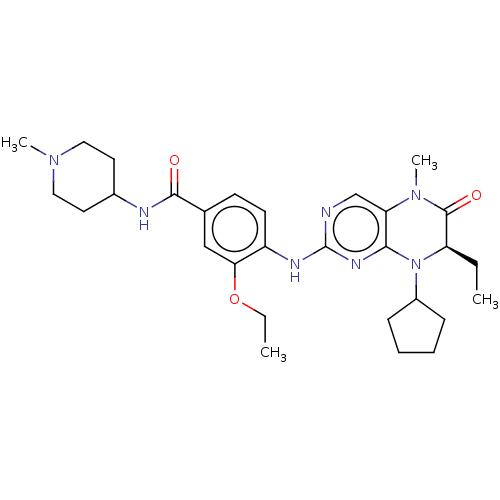 (CHEMBL4464826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526302 (CHEMBL4464826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526302 (CHEMBL4464826) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||