

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50535090 CHEMBL4449815
SMILES: Cc1ccc(cc1)-c1cc2nc[nH]c(=O)c2c(NC[C@H]2CCCNC2)n1
InChI Key: InChIKey=LHOLZQIYHWNEJC-AWEZNQCLSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50535090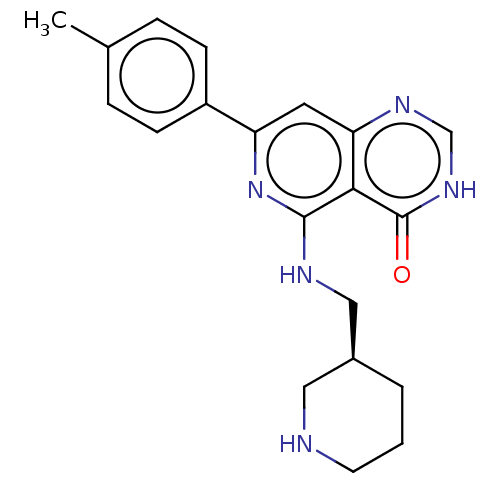 (CHEMBL4449815) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHOK1 cell membranes after 2 hrs by cy3b-dofetilide-based fluorescence polarization assay | Bioorg Med Chem Lett 26: 4606-4612 (2016) Article DOI: 10.1016/j.bmcl.2016.08.070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50535090 (CHEMBL4449815) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant SYK expressed in baculovirus using Biotin-AAAEEIYGEI as substrate after 60 mins by... | Bioorg Med Chem Lett 26: 4606-4612 (2016) Article DOI: 10.1016/j.bmcl.2016.08.070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50535090 (CHEMBL4449815) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of SYK in human B cells assessed as suppression of anti-IgM-induced CD69 surface expression preincubated for 30 mins followed by anti-IgM ... | Bioorg Med Chem Lett 26: 4606-4612 (2016) Article DOI: 10.1016/j.bmcl.2016.08.070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50535090 (CHEMBL4449815) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of N-terminal-6His-tagged full length human Aurora B expressed in baculovirus using 5FAM-PKA-tide as substrate after 120 mins by fluoresce... | Bioorg Med Chem Lett 26: 4606-4612 (2016) Article DOI: 10.1016/j.bmcl.2016.08.070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||