

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCC(CC)CNc1c(cnc2ccc(cc12)-c1ccc(CO)nc1)C(N)=O
InChI Key: InChIKey=UBSRTPHETWULEN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535981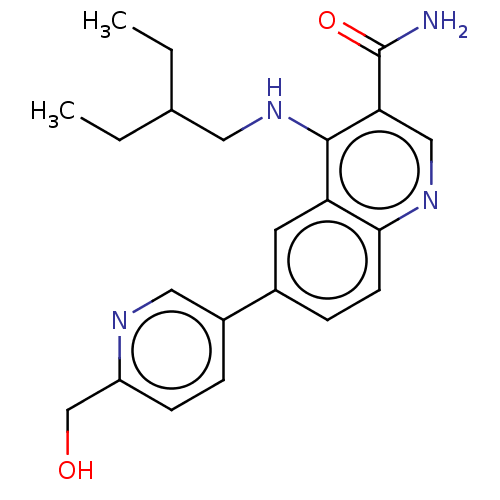 (CHEMBL4588822) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50535981 (CHEMBL4588822) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HT-29 cells assessed as reduction in Chk1 phosphorylation at Ser-345 residue after 60 mins in presence of 4-nitroquinoline... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50535981 (CHEMBL4588822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG by electrophysiology assay | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||