

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50541996 CHEMBL4633982
SMILES: OC(=O)c1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)-c1cn(nn1)-c1ccc(cc1)C(F)(F)F
InChI Key: InChIKey=WYKMWZDXNRNMFC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2A adrenergic receptor (Homo sapiens) | BDBM50541996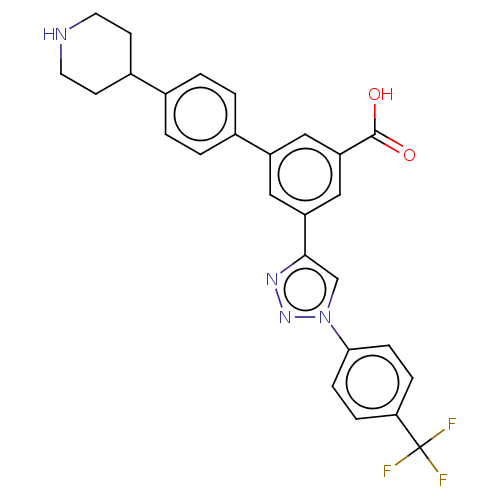 (CHEMBL4633982) | PDB MMDB B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2A receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2C receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to DOR (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2B receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at CB2 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50541996 (CHEMBL4633982) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-... | ACS Med Chem Lett 11: 1281-1286 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y14 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 664 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-... | ACS Med Chem Lett 11: 1281-1286 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50541996 (CHEMBL4633982) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at CB2 receptor (unknown origin) | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50541996 (CHEMBL4633982) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3... | J Med Chem 63: 9563-9589 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||