

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50543961 CHEMBL4647427
SMILES: [O-][N+](=O)c1ccc(cc1C(F)(F)F)S(=O)(=O)Nc1ccc2c(c1)oc(cc2=O)-c1ccccc1
InChI Key: InChIKey=POQDEQXUQQWITL-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Topoisomerase I/II (Homo sapiens (Human)) | BDBM50543961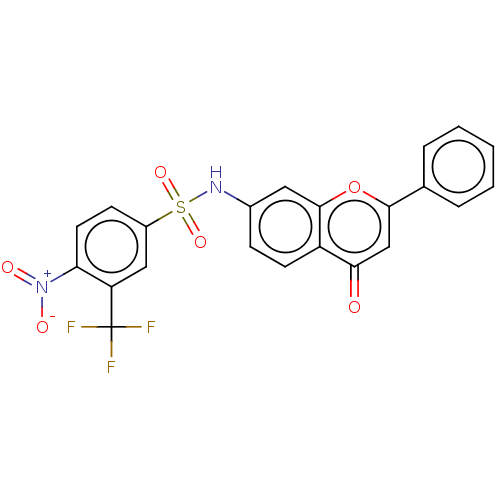 (CHEMBL4647427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||