 Found 15 hits for monomerid = 59206
Found 15 hits for monomerid = 59206 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM59206
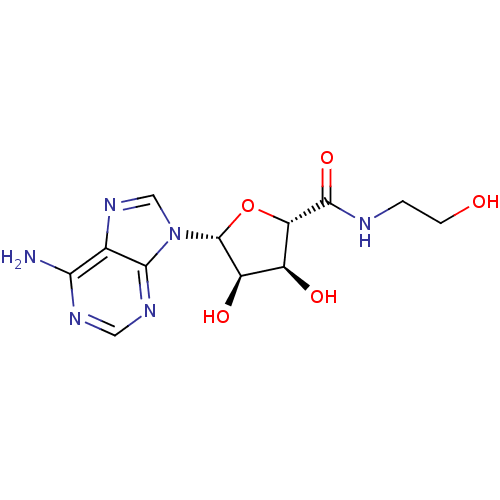
(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
| Assay Description
Binding affinity of ligand at human adenosine receptors expressed in CHO cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of 3[H]R-PIA from human A1 adenosine receptor expressed in CHO cells after 60 mins by Liquid scintillation analysis |
J Med Chem 55: 4297-308 (2012)
Article DOI: 10.1021/jm300095s
BindingDB Entry DOI: 10.7270/Q21C1XXC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]N6-R-phenylisopropyladenosine from human A1A adenosine receptor expressed in CHO cell membranes after 60 mins by scintillation pr... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 41.7 | -10.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
National Institutes of Health
| Assay Description
Binding affinity of ligand at WT and mutant human A2A ARs expressed in COS7 cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [3H]2-[p-(2-carboxyethyl)phenylethylamino]-5-N-ethylcarboxamidoadenosine from human A2A adenosine receptor expressed in HEK293 cell m... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
| Assay Description
Binding affinity of ligand at human adenosine receptors expressed in CHO cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of 3[H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cells after 60 mins by Liquid scintillation analysis |
J Med Chem 55: 4297-308 (2012)
Article DOI: 10.1021/jm300095s
BindingDB Entry DOI: 10.7270/Q21C1XXC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.11E+3 | -7.74 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
National Institutes of Health
| Assay Description
Binding affinity of ligand at WT and mutant human A2A ARs expressed in COS7 cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.87E+3 | -7.38 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
National Institutes of Health
| Assay Description
Binding affinity of ligand at WT and mutant human A2A ARs expressed in COS7 cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human A3 adenosine receptor expressed in CHO cells after 60 mins gamma counter |
J Med Chem 55: 4297-308 (2012)
Article DOI: 10.1021/jm300095s
BindingDB Entry DOI: 10.7270/Q21C1XXC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5-N-methyluronamide from human A3A adenosine receptor expressed in CHO cell membranes after ... |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
| Assay Description
Binding affinity of ligand at human adenosine receptors expressed in CHO cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.33E+4 | -6.65 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
National Institutes of Health
| Assay Description
Binding affinity of ligand at WT and mutant human A2A ARs expressed in COS7 cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |
Adenosine receptors; A2a & A2b
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 948 | n/a | n/a | n/a | n/a |
Medical College of Wisconsin
Curated by ChEMBL
| Assay Description
Agonist activity at human A2B adenosine receptor by cell based assay |
J Med Chem 62: 1502-1522 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01662 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM59206

(Adenosine analog, 11)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)C(=O)NCCO |r| Show InChI InChI=1S/C12H16N6O5/c13-9-5-10(16-3-15-9)18(4-17-5)12-7(21)6(20)8(23-12)11(22)14-1-2-19/h3-4,6-8,12,19-21H,1-2H2,(H,14,22)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 942 | n/a | n/a | n/a | n/a |
National Institutes of Health
| Assay Description
Binding affinity of ligand at human adenosine receptors expressed in CHO cell. |
Chem Biol 12: 237-47 (2005)
Article DOI: 10.1016/j.chembiol.2004.12.010
BindingDB Entry DOI: 10.7270/Q2P26WK5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data