

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600005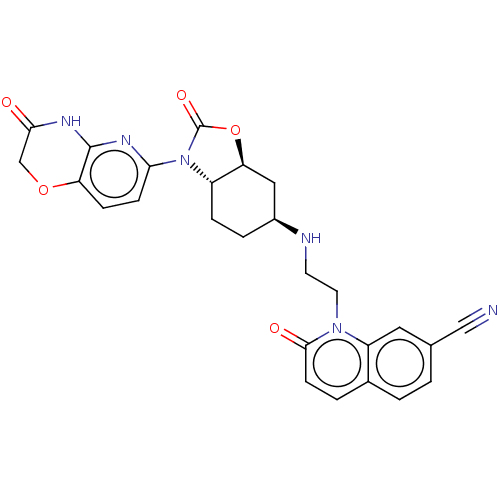 (CHEMBL5195268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600003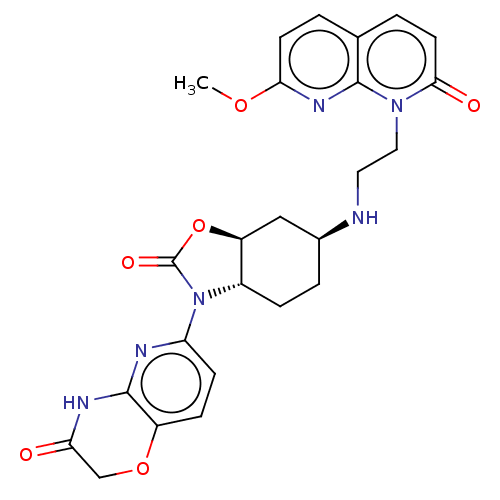 (CHEMBL5201228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50050549 (GSK2140944 | Gepotidacin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600006 (CHEMBL5172158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600007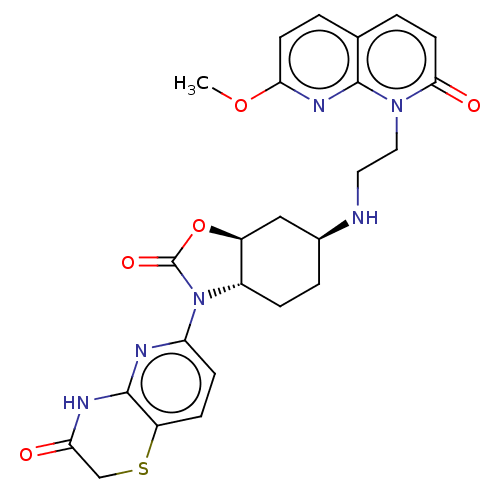 (CHEMBL5182200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574730 ((hydrochloride salt): 3-[4-(Piperidin-4-yl)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600004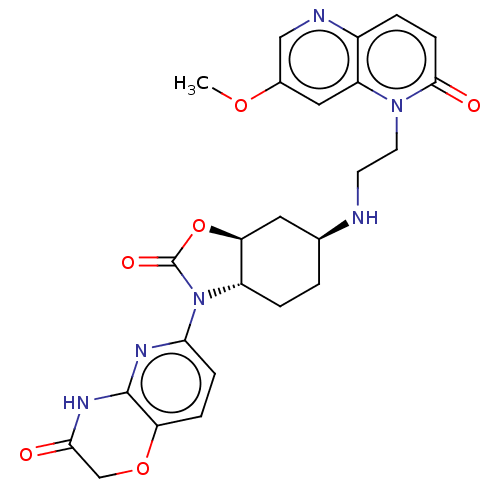 (CHEMBL5184104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574735 ((Hydrochloride Salt): 3-[4-(3-Aminopropylcarbamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574732 (US11459296, Example 40) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574737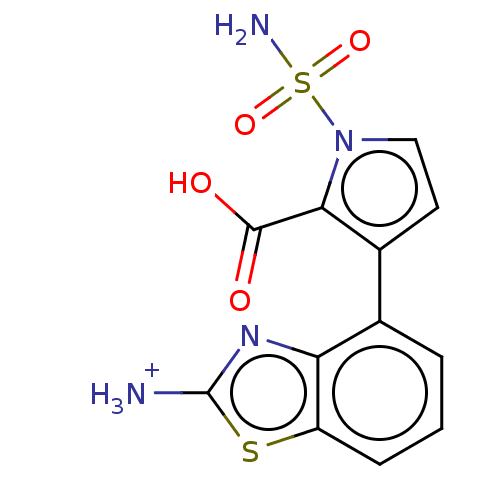 ((Hydrochloride Salt): 3-(2-Amino-1,3-benzothiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600009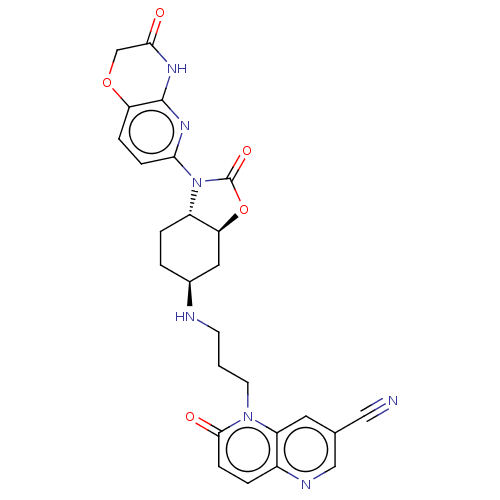 (CHEMBL5177076) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574714 (3-(4-Methanesulfonylphenyl)- 1-sulfamoyl-1H-pyrrol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 444 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574716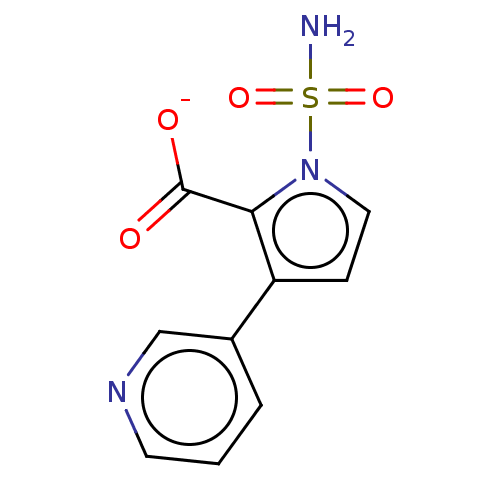 (3-(Pyridin-3-yl)-1-sulfamoyl- 1H-pyrrole-2-carboxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50600008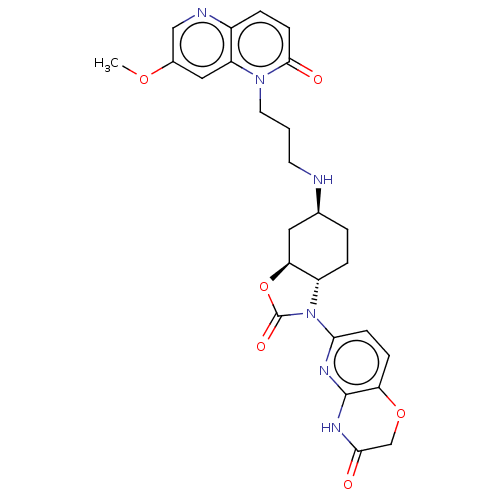 (CHEMBL5198597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574713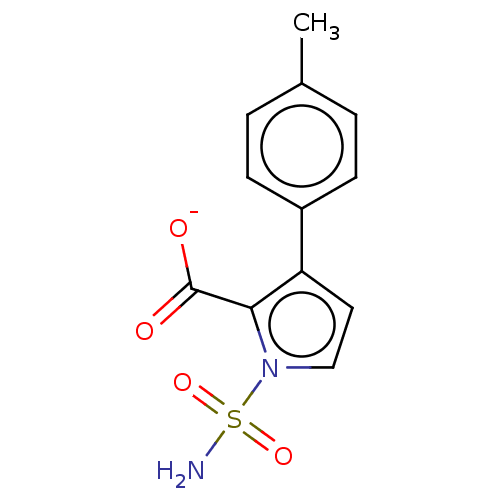 (3-(p-tolyl)-1-sulfamoyl-1H- pyrrole-2-carboxylic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574744 (3-(6-Aminopyridin-3-yl)-2- (1H-tetrazol-5-yl)-1H- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574729 (US11459296, Example 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574726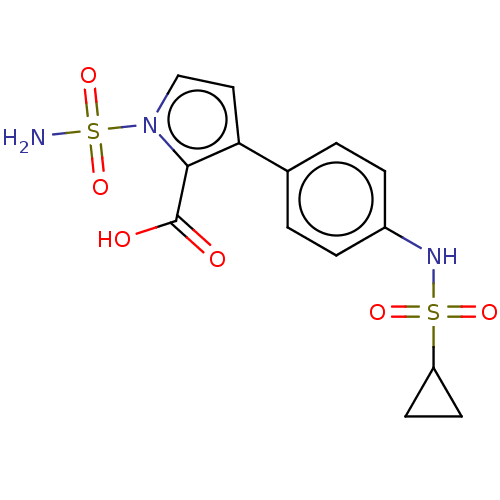 (3-[4- (Cyclopropylsulfonylamino) phenyl]-1-sulfamo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 548 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574727 (3-(6-Acetamido-3-pyridyl)-1- sulfamoyl-pyrrole-2- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574734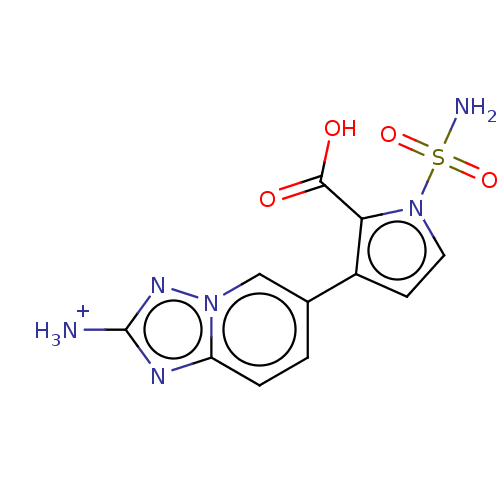 (US11459296, Example 47) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 656 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574721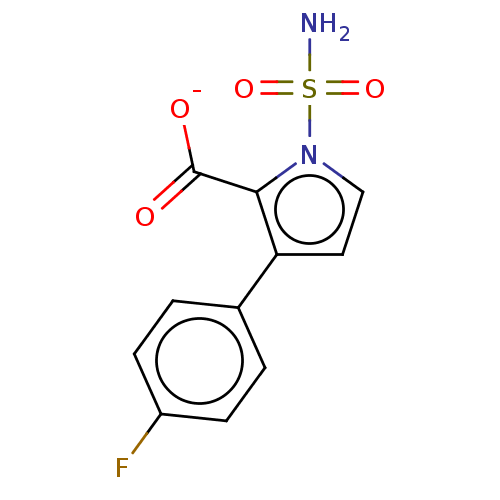 (US11459296, Example 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574712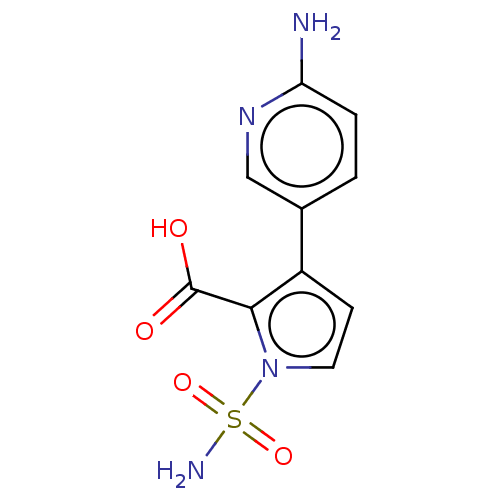 ((Free Acid): 3-(6-Aminopyridin-3-yl)-1-sulfamoyl-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 826 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574740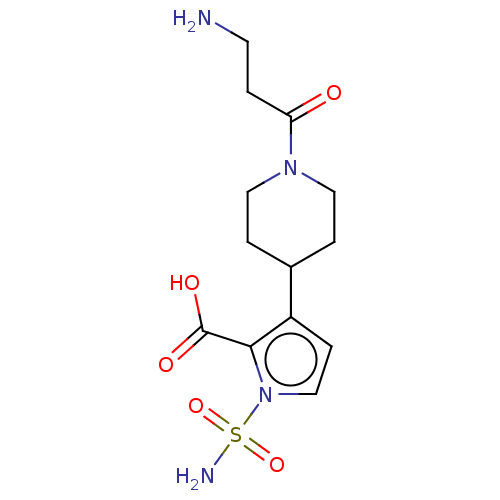 (3-[1-(3-Aminopropanoyl)-4- piperidyl]-1-sulfamoyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 834 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574731 (US11459296, Example 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574743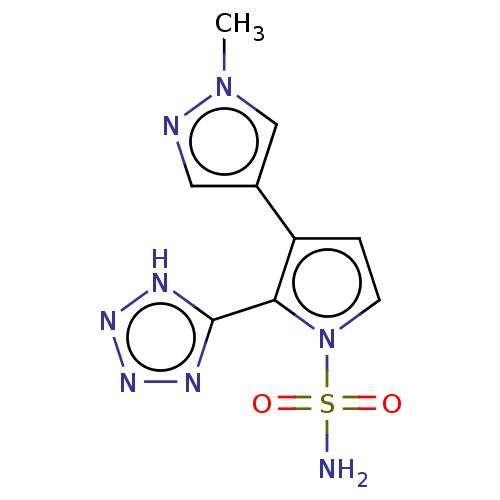 ((free tetrazole): 3-(1-Methyl-1H-pyrazol-4-yl)-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574739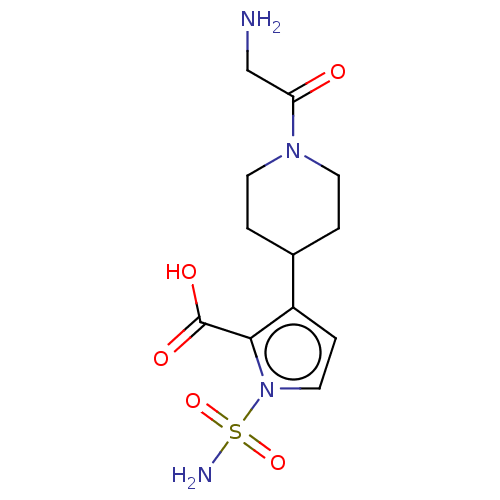 ((free acid): 3-[1-(2-Aminoacetyl)-4-piperidyl]-1-s...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574738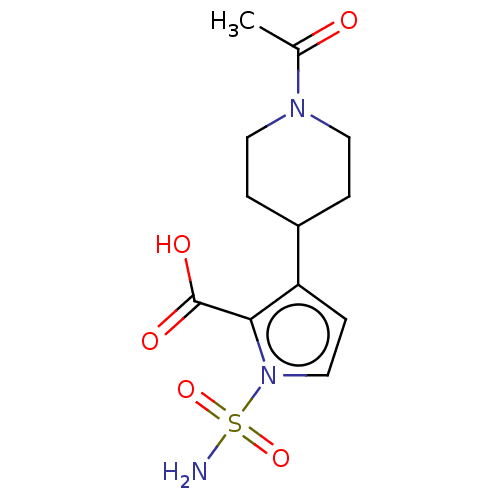 ((free acid): 3-(1-Acetylpiperidin-4-yl)-1-sulfamoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574717 (3-(Pyridin-4-yl)-1-sulfamoyl- 1H-pyrrole-2-carboxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574728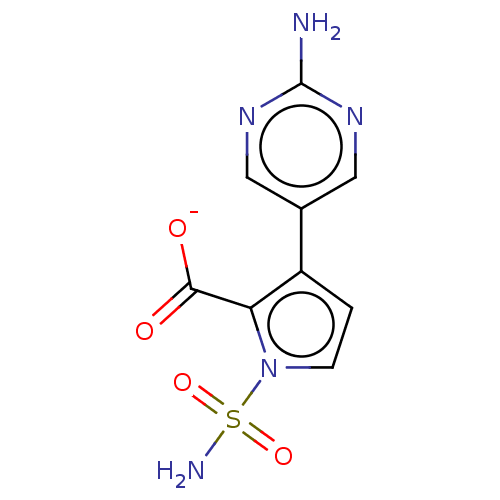 (US11459296, Example 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574722 (3-(3-Fluorophenyl)-1- sulfamoyl-pyrrole-2- carboxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574724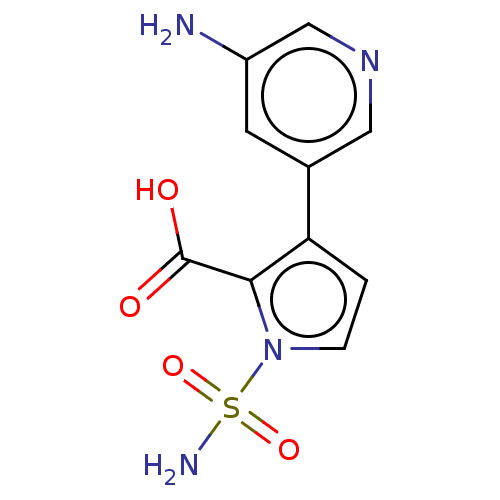 (US11459296, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574742 ((sodium salt): 3-Pyrrol-1-yl-1-sulfamoyl-pyrrole-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574736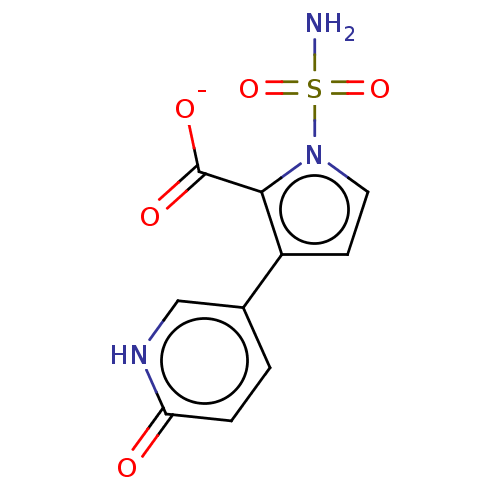 ((Sodium Salt): 3-(6-Oxo-1,6-dihydropyridin-3-yl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574733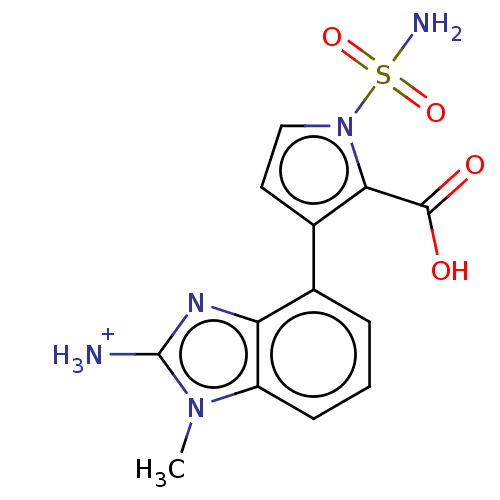 (US11459296, Example 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574715 (3-(1-Methyl-1H-pyrazol-4-yl)- 1-sulfamoyl-1H-pyrro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600011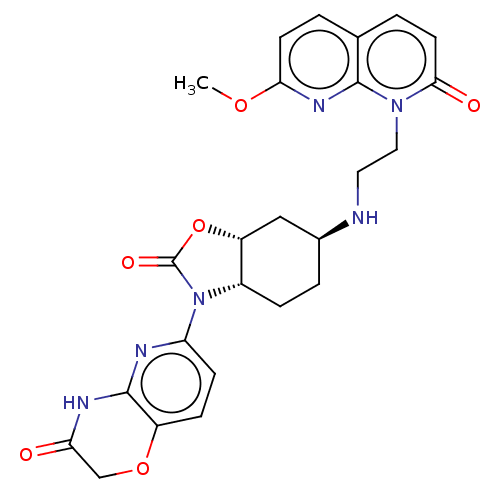 (CHEMBL5205047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574723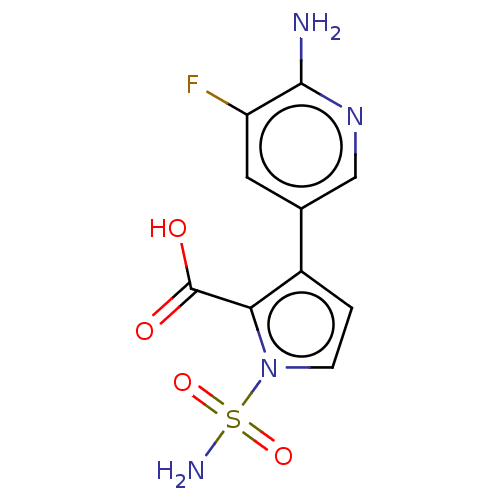 (3-(6-Amino-5-fluoro-3- pyridyl)-1-sulfamoyl-pyrrol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574741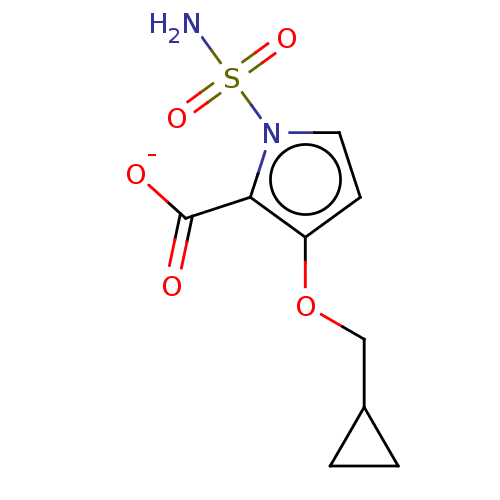 ((Sodium Salt): 3-(Cyclopropylmethoxy)-1-sulfamoyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600007 (CHEMBL5182200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM574718 (US11459296, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of metallo-β-lactamase enzyme function was performed at 37° C. in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 μ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2J106DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600010 (CHEMBL5184352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600003 (CHEMBL5201228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600018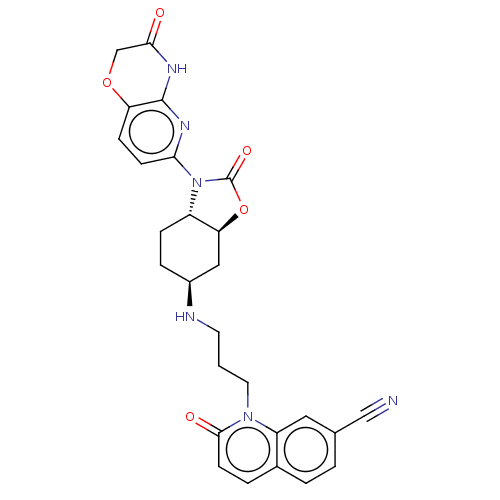 (CHEMBL5199405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600013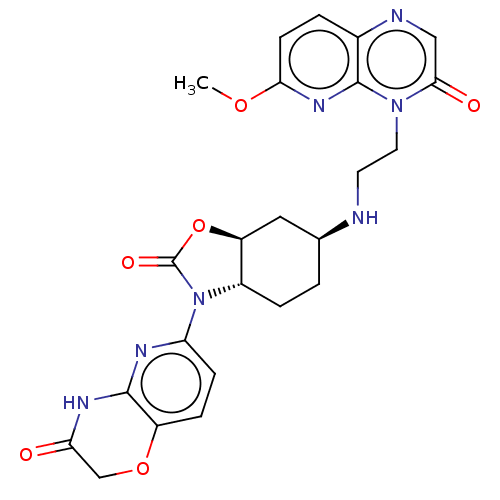 (CHEMBL5194618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600014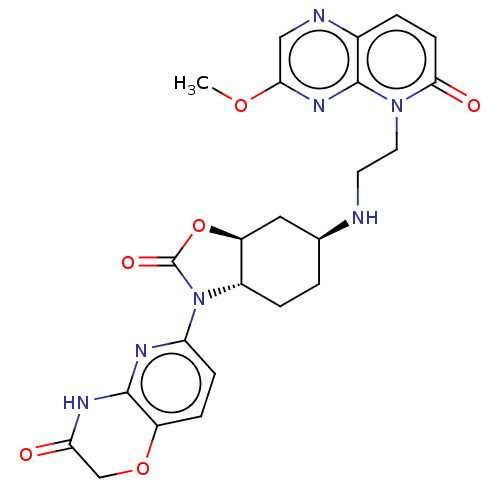 (CHEMBL5172094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600012 (CHEMBL5190832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600017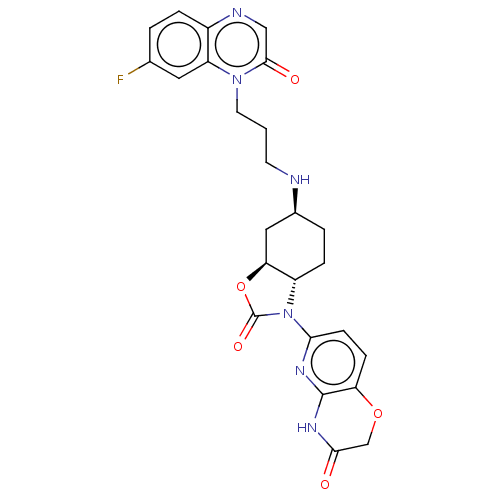 (CHEMBL5195721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50600003 (CHEMBL5201228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50600015 (CHEMBL5206169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128648 BindingDB Entry DOI: 10.7270/Q2MC942Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |