 Found 249 hits with Last Name = 'haegebarth' and Initial = 'a'
Found 249 hits with Last Name = 'haegebarth' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611048

(BAY-091)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1F)C(O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611049

(CHEMBL5280127)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611048

(BAY-091)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1F)C(O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611049

(CHEMBL5280127)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610997
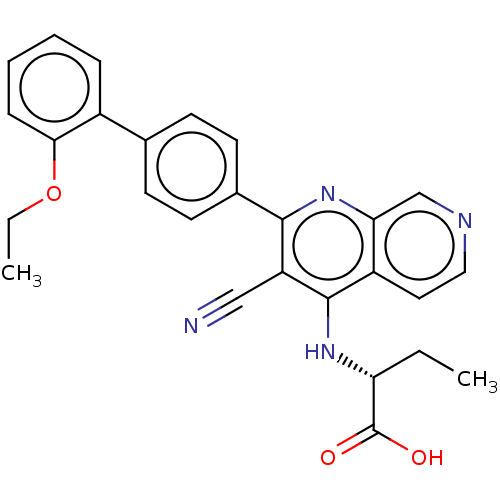
(CHEMBL5276719)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(O)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610997

(CHEMBL5276719)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(O)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611050

(CHEMBL5270765)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cncc(Cl)c2c(N[C@H](CC)C(N)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611048

(BAY-091)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1F)C(O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611046
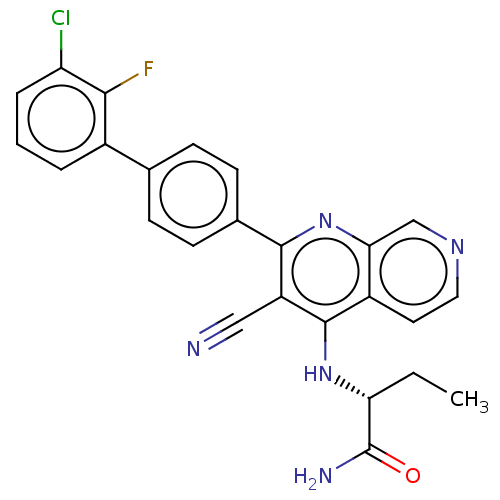
(CHEMBL5290005)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610996

(CHEMBL5271652)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(NC(C)C(O)=O)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611049

(CHEMBL5280127)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611048

(BAY-091)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1F)C(O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508008

(CHEMBL4438379)Show SMILES FC(F)(F)Oc1ccc(cc1)C(=O)N1CCC(CC1)c1ncnc2cc(cnc12)N1CCOCC1 Show InChI InChI=1S/C24H24F3N5O3/c25-24(26,27)35-19-3-1-17(2-4-19)23(33)32-7-5-16(6-8-32)21-22-20(29-15-30-21)13-18(14-28-22)31-9-11-34-12-10-31/h1-4,13-16H,5-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611047

(CHEMBL5277824)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1F)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508013
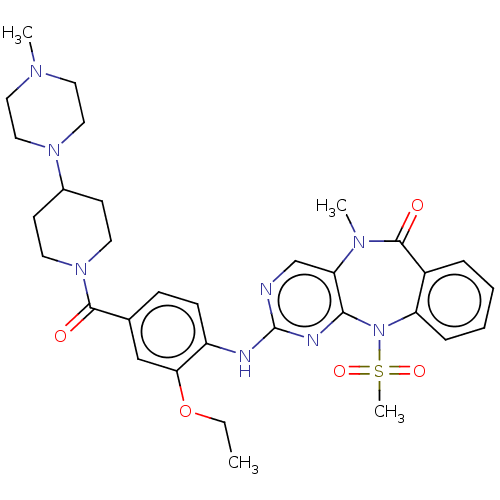
(CHEMBL4541479)Show SMILES CCOc1cc(ccc1Nc1ncc2N(C)C(=O)c3ccccc3N(c2n1)S(C)(=O)=O)C(=O)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C32H40N8O5S/c1-5-45-28-20-22(30(41)39-14-12-23(13-15-39)38-18-16-36(2)17-19-38)10-11-25(28)34-32-33-21-27-29(35-32)40(46(4,43)44)26-9-7-6-8-24(26)31(42)37(27)3/h6-11,20-21,23H,5,12-19H2,1-4H3,(H,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611050

(CHEMBL5270765)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cncc(Cl)c2c(N[C@H](CC)C(N)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610997

(CHEMBL5276719)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(O)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611049

(CHEMBL5280127)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611050

(CHEMBL5270765)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cncc(Cl)c2c(N[C@H](CC)C(N)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611013
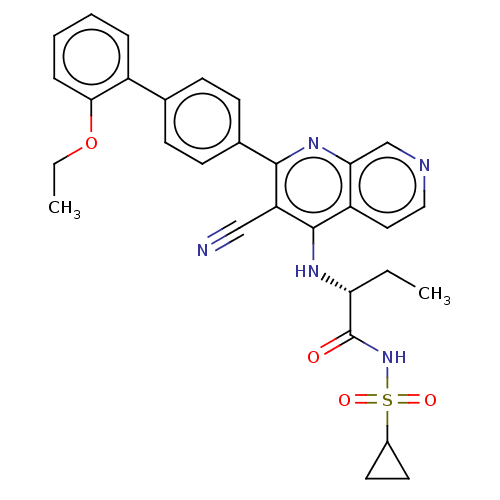
(CHEMBL5288492)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(=O)NS(=O)(=O)C2CC2)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50507989
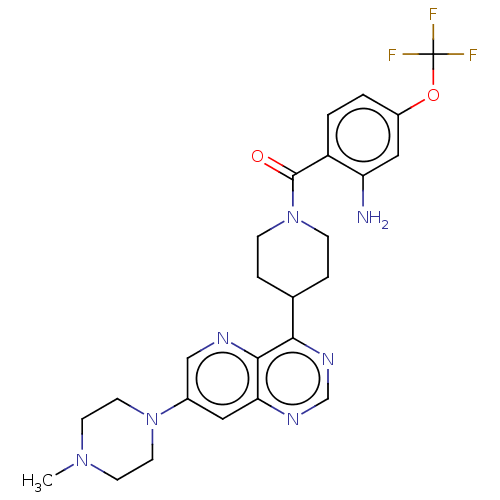
(CHEMBL4445670)Show SMILES CN1CCN(CC1)c1cnc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1N Show InChI InChI=1S/C25H28F3N7O2/c1-33-8-10-34(11-9-33)17-12-21-23(30-14-17)22(32-15-31-21)16-4-6-35(7-5-16)24(36)19-3-2-18(13-20(19)29)37-25(26,27)28/h2-3,12-16H,4-11,29H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611020

(CHEMBL5282226)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1ccccc1F)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611012

(CHEMBL5284796)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(=O)NS(C)(=O)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611023
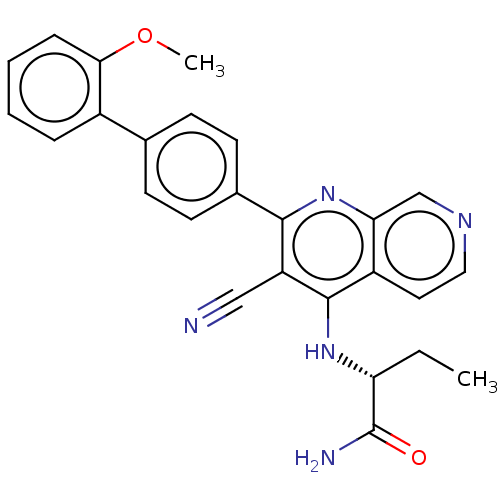
(CHEMBL5273876)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1ccccc1OC)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611043

(CHEMBL5272762)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1ccc(F)cc1OC)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610996

(CHEMBL5271652)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(NC(C)C(O)=O)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50507990
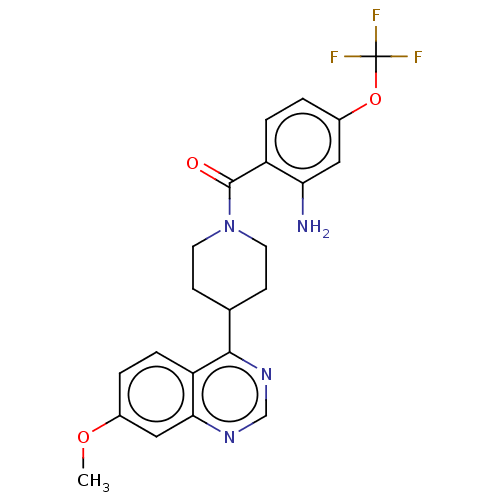
(CHEMBL4469661)Show SMILES COc1ccc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1N Show InChI InChI=1S/C22H21F3N4O3/c1-31-14-2-5-17-19(11-14)27-12-28-20(17)13-6-8-29(9-7-13)21(30)16-4-3-15(10-18(16)26)32-22(23,24)25/h2-5,10-13H,6-9,26H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508009
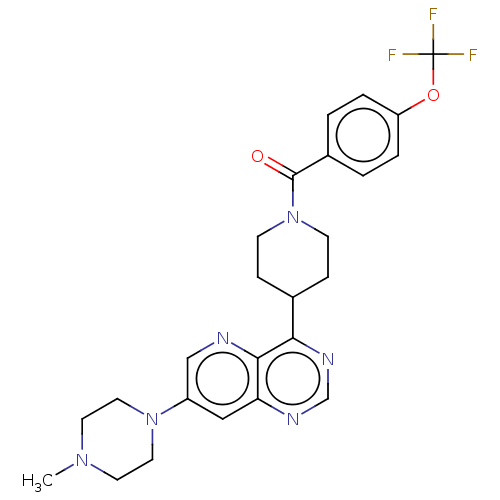
(CHEMBL4550589)Show SMILES CN1CCN(CC1)c1cnc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C25H27F3N6O2/c1-32-10-12-33(13-11-32)19-14-21-23(29-15-19)22(31-16-30-21)17-6-8-34(9-7-17)24(35)18-2-4-20(5-3-18)36-25(26,27)28/h2-5,14-17H,6-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610997

(CHEMBL5276719)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(O)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611033
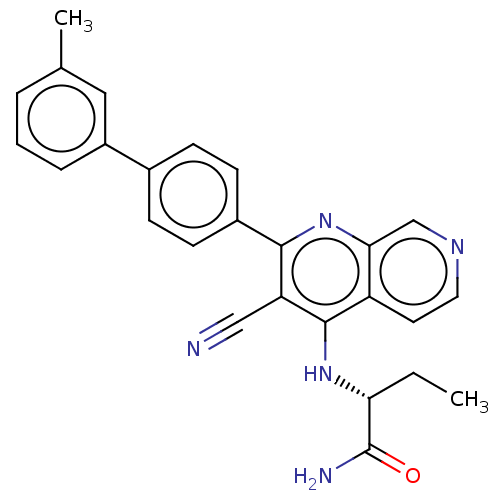
(CHEMBL5265849)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508007

(CHEMBL4542723)Show SMILES CN(C)C1CCN(C1)c1cnc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H29F3N6O2/c1-33(2)19-9-12-35(15-19)20-13-22-24(30-14-20)23(32-16-31-22)17-7-10-34(11-8-17)25(36)18-3-5-21(6-4-18)37-26(27,28)29/h3-6,13-14,16-17,19H,7-12,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50610998
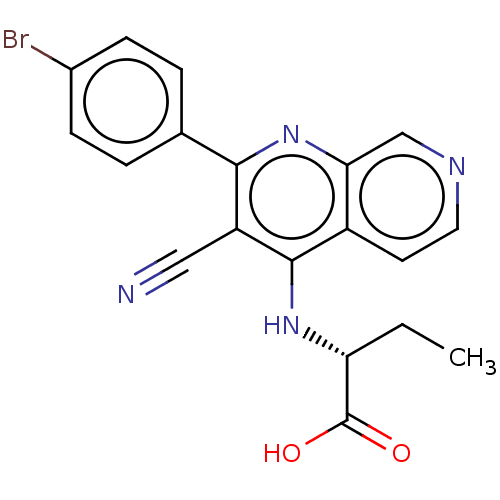
(CHEMBL5274835)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(Br)cc1)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508012

(CHEMBL4526317)Show SMILES CC1(O)CCN(C1)c1cnc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C25H26F3N5O3/c1-24(35)8-11-33(14-24)18-12-20-22(29-13-18)21(31-15-30-20)16-6-9-32(10-7-16)23(34)17-2-4-19(5-3-17)36-25(26,27)28/h2-5,12-13,15-16,35H,6-11,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611046

(CHEMBL5290005)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611047

(CHEMBL5277824)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(C)c1F)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611032
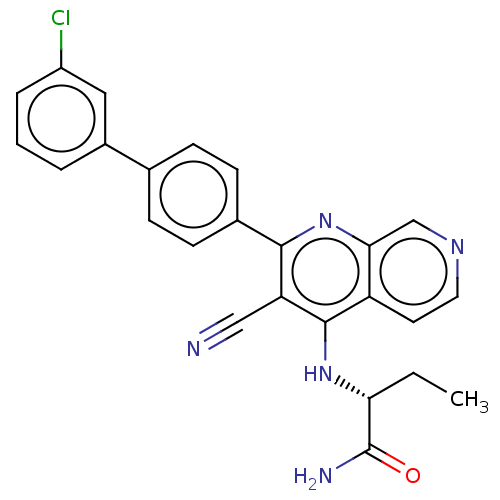
(CHEMBL5275703)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508013

(CHEMBL4541479)Show SMILES CCOc1cc(ccc1Nc1ncc2N(C)C(=O)c3ccccc3N(c2n1)S(C)(=O)=O)C(=O)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C32H40N8O5S/c1-5-45-28-20-22(30(41)39-14-12-23(13-15-39)38-18-16-36(2)17-19-38)10-11-25(28)34-32-33-21-27-29(35-32)40(46(4,43)44)26-9-7-6-8-24(26)31(42)37(27)3/h6-11,20-21,23H,5,12-19H2,1-4H3,(H,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of ERK5 in human SN12C cells transduced with MEF2-responsive transcription element assessed as reduction in MEF2 transcription by measurin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611036
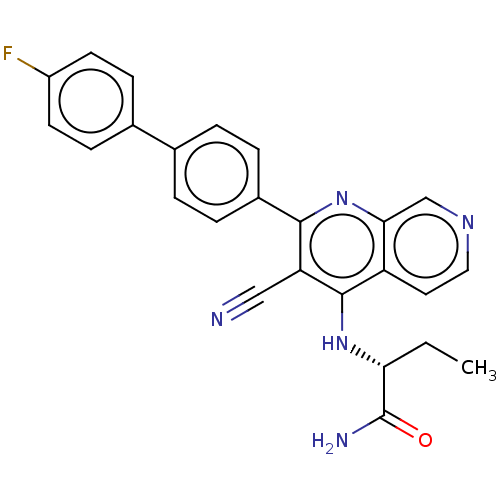
(CHEMBL5288989)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1ccc(F)cc1)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611005
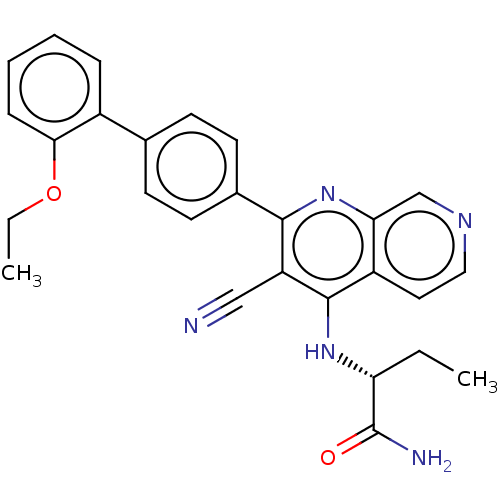
(CHEMBL5273777)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(N)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50507996
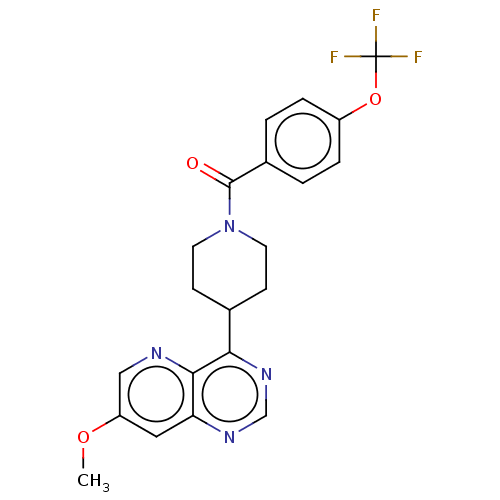
(CHEMBL4441182)Show SMILES COc1cnc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C21H19F3N4O3/c1-30-16-10-17-19(25-11-16)18(27-12-26-17)13-6-8-28(9-7-13)20(29)14-2-4-15(5-3-14)31-21(22,23)24/h2-5,10-13H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611046

(CHEMBL5290005)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1cccc(Cl)c1F)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611022
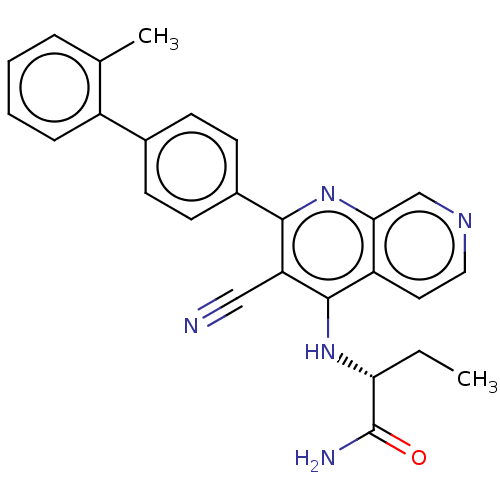
(CHEMBL5286700)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1ccccc1C)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508014
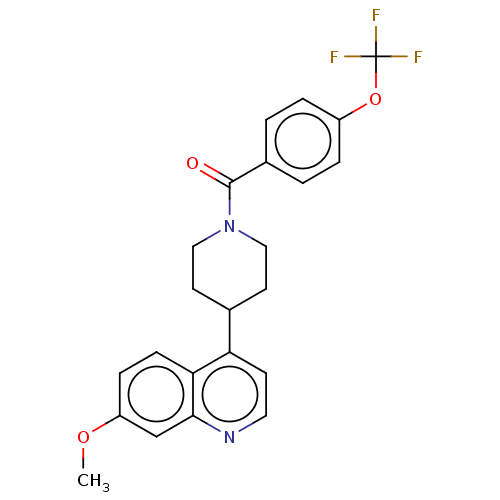
(CHEMBL4450479)Show SMILES COc1ccc2c(ccnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H21F3N2O3/c1-30-18-6-7-20-19(8-11-27-21(20)14-18)15-9-12-28(13-10-15)22(29)16-2-4-17(5-3-16)31-23(24,25)26/h2-8,11,14-15H,9-10,12-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50507989

(CHEMBL4445670)Show SMILES CN1CCN(CC1)c1cnc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1N Show InChI InChI=1S/C25H28F3N7O2/c1-33-8-10-34(11-9-33)17-12-21-23(30-14-17)22(32-15-31-21)16-4-6-35(7-5-16)24(36)19-3-2-18(13-20(19)29)37-25(26,27)28/h2-3,12-16H,4-11,29H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of ERK5 in human SN12C cells transduced with MEF2-responsive transcription element assessed as reduction in MEF2 transcription by measurin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611012

(CHEMBL5284796)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(=O)NS(C)(=O)=O)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611013

(CHEMBL5288492)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2cnccc2c(N[C@H](CC)C(=O)NS(=O)(=O)C2CC2)c1C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50508008

(CHEMBL4438379)Show SMILES FC(F)(F)Oc1ccc(cc1)C(=O)N1CCC(CC1)c1ncnc2cc(cnc12)N1CCOCC1 Show InChI InChI=1S/C24H24F3N5O3/c25-24(26,27)35-19-3-1-17(2-4-19)23(33)32-7-5-16(6-8-32)21-22-20(29-15-30-21)13-18(14-28-22)31-9-11-34-12-10-31/h1-4,13-16H,5-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of ERK5 in human SN12C cells transduced with MEF2-responsive transcription element assessed as reduction in MEF2 transcription by measurin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50507991
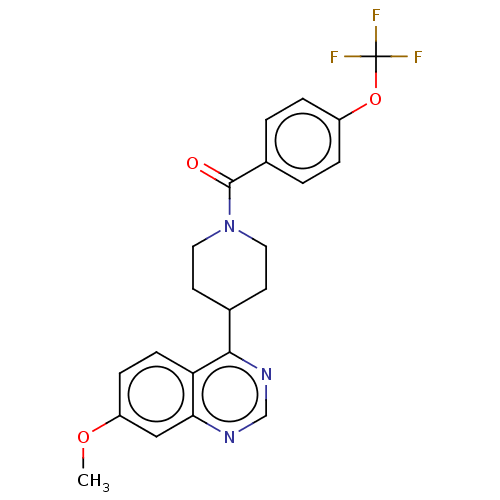
(CHEMBL4450712)Show SMILES COc1ccc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C22H20F3N3O3/c1-30-17-6-7-18-19(12-17)26-13-27-20(18)14-8-10-28(11-9-14)21(29)15-2-4-16(5-3-15)31-22(23,24)25/h2-7,12-14H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha
(Homo sapiens) | BDBM50611021
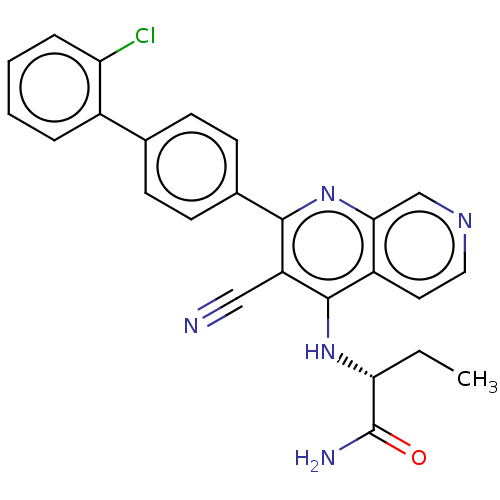
(CHEMBL5277327)Show SMILES CC[C@@H](Nc1c(C#N)c(nc2cnccc12)-c1ccc(cc1)-c1ccccc1Cl)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 7
(Homo sapiens (Human)) | BDBM50507990

(CHEMBL4469661)Show SMILES COc1ccc2c(ncnc2c1)C1CCN(CC1)C(=O)c1ccc(OC(F)(F)F)cc1N Show InChI InChI=1S/C22H21F3N4O3/c1-31-14-2-5-17-19(11-14)27-12-28-20(17)13-6-8-29(9-7-13)21(30)16-4-3-15(10-18(16)26)32-22(23,24)25/h2-5,10-13H,6-9,26H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of ERK5 in human SN12C cells transduced with MEF2-responsive transcription element assessed as reduction in MEF2 transcription by measurin... |
J Med Chem 62: 928-940 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01606
BindingDB Entry DOI: 10.7270/Q2FN19H1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data