

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM16175 (CHEMBL108468 | UKI-1 | ethyl 4-(3-carbamimidoyl-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | -42.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16175 (CHEMBL108468 | UKI-1 | ethyl 4-(3-carbamimidoyl-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | -36.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM16174 (3-[3-(4-beta-alanylpiperazin-1-yl)-3-oxo-2-({[2,4,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 600 | -35.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16174 (3-[3-(4-beta-alanylpiperazin-1-yl)-3-oxo-2-({[2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 640 | -35.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16176 (3-(1-adamantyl)-1-[(4-carbamimidamidophenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 2.40E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM16175 (CHEMBL108468 | UKI-1 | ethyl 4-(3-carbamimidoyl-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16173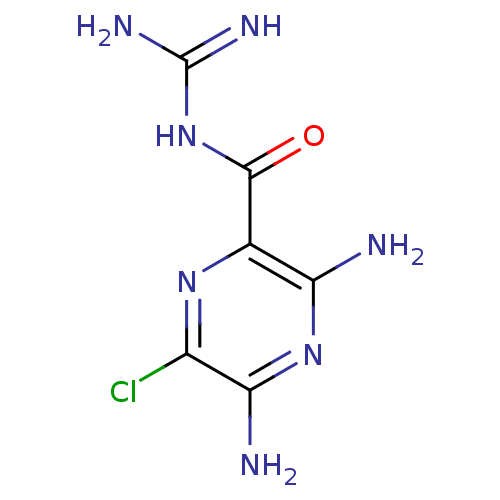 (3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5.30E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM16174 (3-[3-(4-beta-alanylpiperazin-1-yl)-3-oxo-2-({[2,4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.70E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM16173 (3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM772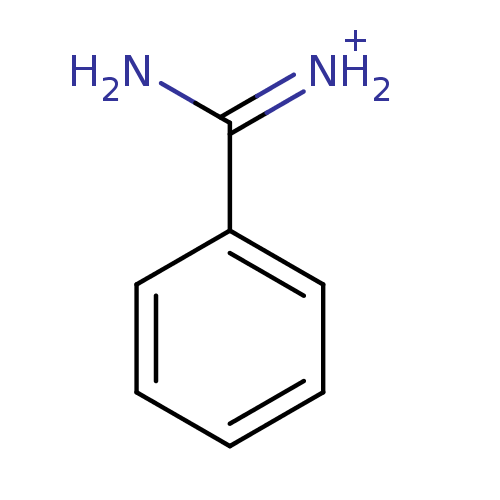 (Benzamidine | CHEMBL79897 | [amino(phenyl)methylid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 3.90E+4 | -25.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM16176 (3-(1-adamantyl)-1-[(4-carbamimidamidophenyl)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 4.60E+4 | -24.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM772 (Benzamidine | CHEMBL79897 | [amino(phenyl)methylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.80E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM772 (Benzamidine | CHEMBL79897 | [amino(phenyl)methylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM16176 (3-(1-adamantyl)-1-[(4-carbamimidamidophenyl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM16173 (3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+6 | >-17.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Max-Planck-Institut fuer Biochemie | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used in t... | J Mol Biol 301: 465-75 (2000) Article DOI: 10.1006/jmbi.2000.3966 BindingDB Entry DOI: 10.7270/Q2445JRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||