 Found 119 hits with Last Name = 'kittaka' and Initial = 'a'
Found 119 hits with Last Name = 'kittaka' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411176

(CHEMBL383850)Show SMILES CCC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-6-8-24-20(4)29(33)34-28(24)15-18(2)25-12-13-26-21(9-7-14-30(25,26)5)10-11-22-16-23(31)17-27(32)19(22)3/h10-11,18,23-28,31-32H,3-4,6-9,12-17H2,1-2,5H3/b21-10+,22-11-/t18-,23-,24+,25-,26+,27+,28+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411164
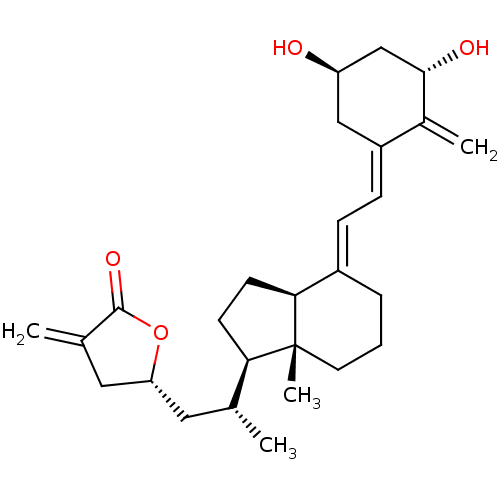
(CHEMBL386412)Show SMILES C[C@H](C[C@@H]1CC(=C)C(=O)O1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C27H38O4/c1-16(12-22-13-17(2)26(30)31-22)23-9-10-24-19(6-5-11-27(23,24)4)7-8-20-14-21(28)15-25(29)18(20)3/h7-8,16,21-25,28-29H,2-3,5-6,9-15H2,1,4H3/b19-7+,20-8-/t16-,21-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411106

(CHEMBL387098)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)C11CCCC1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C31H44O4/c1-19(16-28-31(14-5-6-15-31)21(3)29(34)35-28)25-11-12-26-22(8-7-13-30(25,26)4)9-10-23-17-24(32)18-27(33)20(23)2/h9-10,19,24-28,32-33H,2-3,5-8,11-18H2,1,4H3/b22-9+,23-10-/t19-,24-,25-,26+,27+,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411105
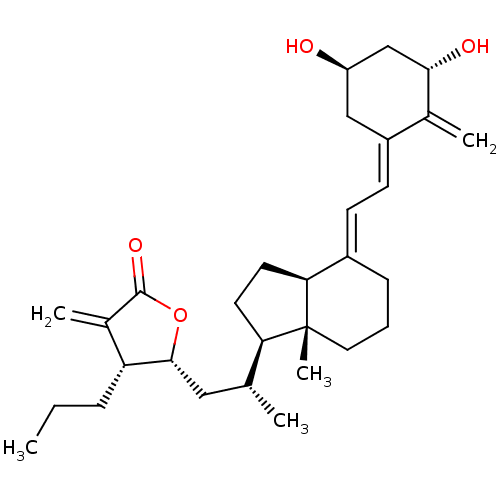
(CHEMBL216037)Show SMILES CCC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-6-8-24-20(4)29(33)34-28(24)15-18(2)25-12-13-26-21(9-7-14-30(25,26)5)10-11-22-16-23(31)17-27(32)19(22)3/h10-11,18,23-28,31-32H,3-4,6-9,12-17H2,1-2,5H3/b21-10+,22-11-/t18-,23-,24-,25-,26+,27+,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411143

(CHEMBL384614)Show SMILES CCC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-6-8-24-20(4)29(33)34-28(24)15-18(2)25-12-13-26-21(9-7-14-30(25,26)5)10-11-22-16-23(31)17-27(32)19(22)3/h10-11,18,23-28,31-32H,3-4,6-9,12-17H2,1-2,5H3/b21-10+,22-11-/t18-,23-,24+,25-,26+,27+,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411137

(CHEMBL383910)Show SMILES CCC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-6-8-24-20(4)29(33)34-28(24)15-18(2)25-12-13-26-21(9-7-14-30(25,26)5)10-11-22-16-23(31)17-27(32)19(22)3/h10-11,18,23-28,31-32H,3-4,6-9,12-17H2,1-2,5H3/b21-10+,22-11-/t18-,23-,24-,25-,26+,27+,28+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411132
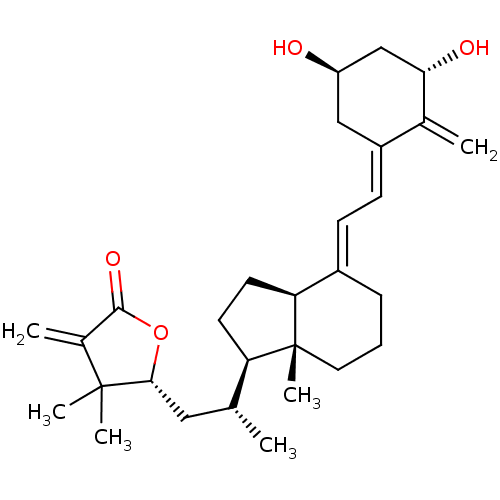
(CHEMBL213868)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)C1(C)C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C29H42O4/c1-17(14-26-28(4,5)19(3)27(32)33-26)23-11-12-24-20(8-7-13-29(23,24)6)9-10-21-15-22(30)16-25(31)18(21)2/h9-10,17,22-26,30-31H,2-3,7-8,11-16H2,1,4-6H3/b20-9+,21-10-/t17-,22-,23-,24+,25+,26-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411161

(CHEMBL385205)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](OCCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O6/c1-18(16-27-19(2)20(3)30(35)37-27)24-11-12-25-22(8-6-13-31(24,25)5)9-10-23-17-26(33)29(28(34)21(23)4)36-15-7-14-32/h9-10,18-19,24-29,32-34H,3-4,6-8,11-17H2,1-2,5H3/b22-9+,23-10-/t18-,19-,24-,25+,26-,27+,28-,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411150

(CHEMBL387220)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](OCCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O6/c1-18(16-27-19(2)20(3)30(35)37-27)24-11-12-25-22(8-6-13-31(24,25)5)9-10-23-17-26(33)29(28(34)21(23)4)36-15-7-14-32/h9-10,18-19,24-29,32-34H,3-4,6-8,11-17H2,1-2,5H3/b22-9+,23-10-/t18-,19-,24-,25+,26-,27-,28-,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411133
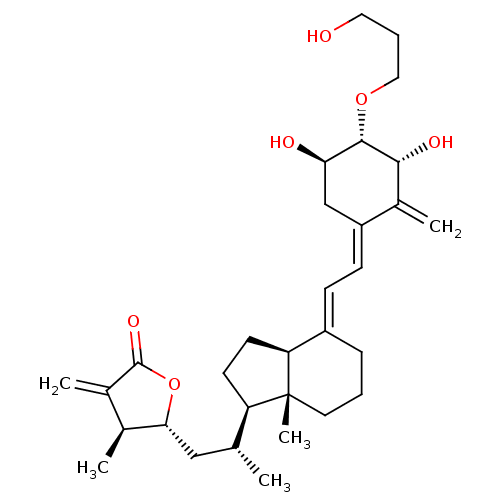
(CHEMBL385705)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](OCCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O6/c1-18(16-27-19(2)20(3)30(35)37-27)24-11-12-25-22(8-6-13-31(24,25)5)9-10-23-17-26(33)29(28(34)21(23)4)36-15-7-14-32/h9-10,18-19,24-29,32-34H,3-4,6-8,11-17H2,1-2,5H3/b22-9+,23-10-/t18-,19+,24-,25+,26-,27-,28-,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411115
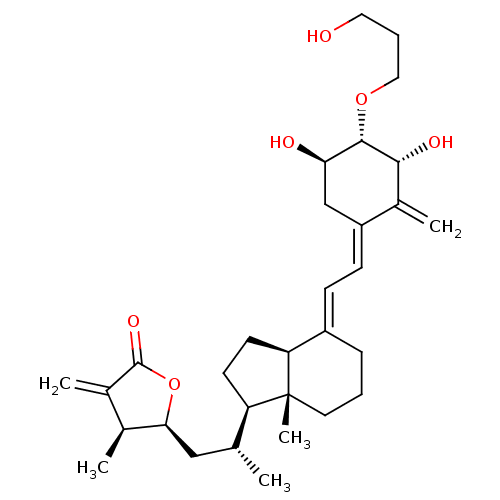
(CHEMBL217924)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](OCCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O6/c1-18(16-27-19(2)20(3)30(35)37-27)24-11-12-25-22(8-6-13-31(24,25)5)9-10-23-17-26(33)29(28(34)21(23)4)36-15-7-14-32/h9-10,18-19,24-29,32-34H,3-4,6-8,11-17H2,1-2,5H3/b22-9+,23-10-/t18-,19+,24-,25+,26-,27+,28-,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411159

(CHEMBL214978)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](CCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O5/c1-18(16-28-19(2)20(3)30(35)36-28)25-12-13-26-22(8-6-14-31(25,26)5)10-11-23-17-27(33)24(9-7-15-32)29(34)21(23)4/h10-11,18-19,24-29,32-34H,3-4,6-9,12-17H2,1-2,5H3/b22-10+,23-11-/t18-,19-,24+,25-,26+,27-,28+,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411157

(CHEMBL215164)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](CCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O5/c1-18(16-28-19(2)20(3)30(35)36-28)25-12-13-26-22(8-6-14-31(25,26)5)10-11-23-17-27(33)24(9-7-15-32)29(34)21(23)4/h10-11,18-19,24-29,32-34H,3-4,6-9,12-17H2,1-2,5H3/b22-10+,23-11-/t18-,19+,24+,25-,26+,27-,28-,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411158

(CHEMBL385707)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](CCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O5/c1-18(16-28-19(2)20(3)30(35)36-28)25-12-13-26-22(8-6-14-31(25,26)5)10-11-23-17-27(33)24(9-7-15-32)29(34)21(23)4/h10-11,18-19,24-29,32-34H,3-4,6-9,12-17H2,1-2,5H3/b22-10+,23-11-/t18-,19-,24+,25-,26+,27-,28-,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411124

(CHEMBL217385)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](CCCO)[C@H](O)C1=C Show InChI InChI=1S/C31H46O5/c1-18(16-28-19(2)20(3)30(35)36-28)25-12-13-26-22(8-6-14-31(25,26)5)10-11-23-17-27(33)24(9-7-15-32)29(34)21(23)4/h10-11,18-19,24-29,32-34H,3-4,6-9,12-17H2,1-2,5H3/b22-10+,23-11-/t18-,19+,24+,25-,26+,27-,28+,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411169

(CHEMBL383902)Show SMILES C[C@H](C[C@H]1CC(=C)C(=O)O1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C27H38O4/c1-16(12-22-13-17(2)26(30)31-22)23-9-10-24-19(6-5-11-27(23,24)4)7-8-20-14-21(28)15-25(29)18(20)3/h7-8,16,21-25,28-29H,2-3,5-6,9-15H2,1,4H3/b19-7+,20-8-/t16-,21-,22+,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411174
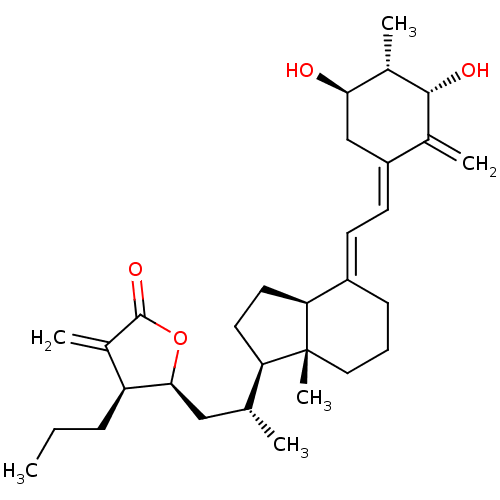
(CHEMBL384929)Show SMILES CCC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-7-9-24-20(4)30(34)35-28(24)16-18(2)25-13-14-26-22(10-8-15-31(25,26)6)11-12-23-17-27(32)21(5)29(33)19(23)3/h11-12,18,21,24-29,32-33H,3-4,7-10,13-17H2,1-2,5-6H3/b22-11+,23-12-/t18-,21+,24+,25-,26+,27-,28+,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411182

(CHEMBL387281)Show SMILES CCC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](OCCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O6/c1-6-9-25-22(4)32(37)39-29(25)18-20(2)26-13-14-27-23(10-7-15-33(26,27)5)11-12-24-19-28(35)31(30(36)21(24)3)38-17-8-16-34/h11-12,20,25-31,34-36H,3-4,6-10,13-19H2,1-2,5H3/b23-11+,24-12-/t20-,25-,26-,27+,28-,29-,30-,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411183
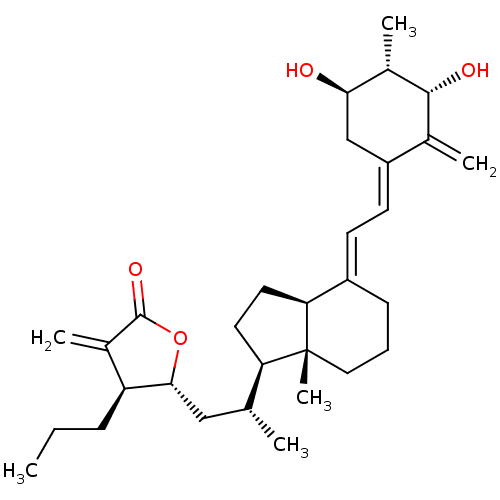
(CHEMBL217447)Show SMILES CCC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-7-9-24-20(4)30(34)35-28(24)16-18(2)25-13-14-26-22(10-8-15-31(25,26)6)11-12-23-17-27(32)21(5)29(33)19(23)3/h11-12,18,21,24-29,32-33H,3-4,7-10,13-17H2,1-2,5-6H3/b22-11+,23-12-/t18-,21+,24+,25-,26+,27-,28-,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411184
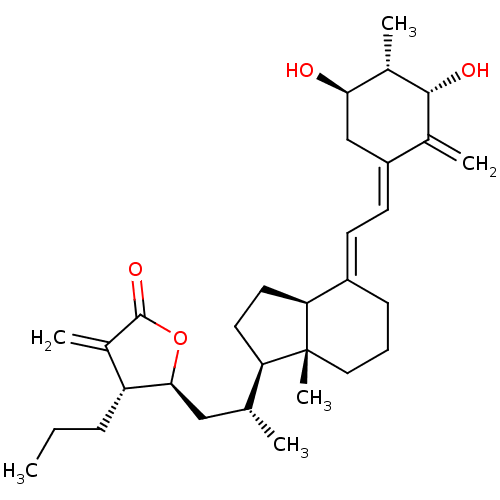
(CHEMBL215239)Show SMILES CCC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-7-9-24-20(4)30(34)35-28(24)16-18(2)25-13-14-26-22(10-8-15-31(25,26)6)11-12-23-17-27(32)21(5)29(33)19(23)3/h11-12,18,21,24-29,32-33H,3-4,7-10,13-17H2,1-2,5-6H3/b22-11+,23-12-/t18-,21+,24-,25-,26+,27-,28+,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411110

(CHEMBL215306)Show SMILES CCC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](CCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O5/c1-6-9-25-22(4)32(37)38-30(25)18-20(2)27-14-15-28-23(10-7-16-33(27,28)5)12-13-24-19-29(35)26(11-8-17-34)31(36)21(24)3/h12-13,20,25-31,34-36H,3-4,6-11,14-19H2,1-2,5H3/b23-12+,24-13-/t20-,25-,26+,27-,28+,29-,30-,31-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411111
(CHEMBL385342)Show SMILES CCC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](CCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O5/c1-6-9-25-22(4)32(37)38-30(25)18-20(2)27-14-15-28-23(10-7-16-33(27,28)5)12-13-24-19-29(35)26(11-8-17-34)31(36)21(24)3/h12-13,20,25-31,34-36H,3-4,6-11,14-19H2,1-2,5H3/b23-12+,24-13-/t20-,25+,26+,27-,28+,29-,30+,31-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411120

(CHEMBL218565)Show SMILES CCC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](OCCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O6/c1-6-9-25-22(4)32(37)39-29(25)18-20(2)26-13-14-27-23(10-7-15-33(26,27)5)11-12-24-19-28(35)31(30(36)21(24)3)38-17-8-16-34/h11-12,20,25-31,34-36H,3-4,6-10,13-19H2,1-2,5H3/b23-11+,24-12-/t20-,25-,26-,27+,28-,29+,30-,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411126
(CHEMBL384370)Show SMILES CCC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](CCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O5/c1-6-9-25-22(4)32(37)38-30(25)18-20(2)27-14-15-28-23(10-7-16-33(27,28)5)12-13-24-19-29(35)26(11-8-17-34)31(36)21(24)3/h12-13,20,25-31,34-36H,3-4,6-11,14-19H2,1-2,5H3/b23-12+,24-13-/t20-,25+,26+,27-,28+,29-,30-,31-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411127

(CHEMBL214500)Show SMILES CCC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](CCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O5/c1-6-9-25-22(4)32(37)38-30(25)18-20(2)27-14-15-28-23(10-7-16-33(27,28)5)12-13-24-19-29(35)26(11-8-17-34)31(36)21(24)3/h12-13,20,25-31,34-36H,3-4,6-11,14-19H2,1-2,5H3/b23-12+,24-13-/t20-,25-,26+,27-,28+,29-,30+,31-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411136
(CHEMBL385926)Show SMILES CCC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-7-9-24-20(4)30(34)35-28(24)16-18(2)25-13-14-26-22(10-8-15-31(25,26)6)11-12-23-17-27(32)21(5)29(33)19(23)3/h11-12,18,21,24-29,32-33H,3-4,7-10,13-17H2,1-2,5-6H3/b22-11+,23-12-/t18-,21+,24-,25-,26+,27-,28-,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411142

(CHEMBL217417)Show SMILES CCC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](OCCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O6/c1-6-9-25-22(4)32(37)39-29(25)18-20(2)26-13-14-27-23(10-7-15-33(26,27)5)11-12-24-19-28(35)31(30(36)21(24)3)38-17-8-16-34/h11-12,20,25-31,34-36H,3-4,6-10,13-19H2,1-2,5H3/b23-11+,24-12-/t20-,25+,26-,27+,28-,29+,30-,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411165

(CHEMBL215360)Show SMILES CCC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](OCCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C33H50O6/c1-6-9-25-22(4)32(37)39-29(25)18-20(2)26-13-14-27-23(10-7-15-33(26,27)5)11-12-24-19-28(35)31(30(36)21(24)3)38-17-8-16-34/h11-12,20,25-31,34-36H,3-4,6-10,13-19H2,1-2,5H3/b23-11+,24-12-/t20-,25+,26-,27+,28-,29-,30-,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411167
(CHEMBL215173)Show SMILES CC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-7-23-19(4)29(33)34-27(23)15-17(2)24-12-13-25-21(9-8-14-30(24,25)6)10-11-22-16-26(31)20(5)28(32)18(22)3/h10-11,17,20,23-28,31-32H,3-4,7-9,12-16H2,1-2,5-6H3/b21-10+,22-11-/t17-,20+,23-,24-,25+,26-,27+,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411104

(CHEMBL217335)Show SMILES CCCC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O4/c1-7-8-11-25-21(4)31(35)36-29(25)17-19(2)26-14-15-27-23(10-9-16-32(26,27)6)12-13-24-18-28(33)22(5)30(34)20(24)3/h12-13,19,22,25-30,33-34H,3-4,7-11,14-18H2,1-2,5-6H3/b23-12+,24-13-/t19-,22+,25+,26-,27+,28-,29-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411149

(CHEMBL215518)Show SMILES CC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-7-23-19(4)29(33)34-27(23)15-17(2)24-12-13-25-21(9-8-14-30(24,25)6)10-11-22-16-26(31)20(5)28(32)18(22)3/h10-11,17,20,23-28,31-32H,3-4,7-9,12-16H2,1-2,5-6H3/b21-10+,22-11-/t17-,20+,23+,24-,25+,26-,27-,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411102

(CHEMBL384538)Show SMILES CCCC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O4/c1-7-8-11-25-21(4)31(35)36-29(25)17-19(2)26-14-15-27-23(10-9-16-32(26,27)6)12-13-24-18-28(33)22(5)30(34)20(24)3/h12-13,19,22,25-30,33-34H,3-4,7-11,14-18H2,1-2,5-6H3/b23-12+,24-13-/t19-,22+,25-,26-,27+,28-,29+,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411135

(CHEMBL386623)Show SMILES CCCC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O4/c1-7-8-11-25-21(4)31(35)36-29(25)17-19(2)26-14-15-27-23(10-9-16-32(26,27)6)12-13-24-18-28(33)22(5)30(34)20(24)3/h12-13,19,22,25-30,33-34H,3-4,7-11,14-18H2,1-2,5-6H3/b23-12+,24-13-/t19-,22+,25-,26-,27+,28-,29-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411154

(CHEMBL214469)Show SMILES CCCC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O4/c1-7-8-11-25-21(4)31(35)36-29(25)17-19(2)26-14-15-27-23(10-9-16-32(26,27)6)12-13-24-18-28(33)22(5)30(34)20(24)3/h12-13,19,22,25-30,33-34H,3-4,7-11,14-18H2,1-2,5-6H3/b23-12+,24-13-/t19-,22+,25+,26-,27+,28-,29+,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411173

(CHEMBL215968)Show SMILES CC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-7-23-19(4)29(33)34-27(23)15-17(2)24-12-13-25-21(9-8-14-30(24,25)6)10-11-22-16-26(31)20(5)28(32)18(22)3/h10-11,17,20,23-28,31-32H,3-4,7-9,12-16H2,1-2,5-6H3/b21-10+,22-11-/t17-,20+,23-,24-,25+,26-,27-,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411163

(CHEMBL217030)Show SMILES CC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](C)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C30H44O4/c1-7-23-19(4)29(33)34-27(23)15-17(2)24-12-13-25-21(9-8-14-30(24,25)6)10-11-22-16-26(31)20(5)28(32)18(22)3/h10-11,17,20,23-28,31-32H,3-4,7-9,12-16H2,1-2,5-6H3/b21-10+,22-11-/t17-,20+,23+,24-,25+,26-,27+,28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411144

(CHEMBL384020)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](C)[C@H](O)C1=C Show InChI InChI=1S/C29H42O4/c1-16(14-26-17(2)18(3)28(32)33-26)23-11-12-24-21(8-7-13-29(23,24)6)9-10-22-15-25(30)20(5)27(31)19(22)4/h9-10,16-17,20,23-27,30-31H,3-4,7-8,11-15H2,1-2,5-6H3/b21-9+,22-10-/t16-,17+,20+,23-,24+,25-,26+,27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411128

(CHEMBL215806)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](C)[C@H](O)C1=C Show InChI InChI=1S/C29H42O4/c1-16(14-26-17(2)18(3)28(32)33-26)23-11-12-24-21(8-7-13-29(23,24)6)9-10-22-15-25(30)20(5)27(31)19(22)4/h9-10,16-17,20,23-27,30-31H,3-4,7-8,11-15H2,1-2,5-6H3/b21-9+,22-10-/t16-,17-,20+,23-,24+,25-,26+,27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411180

(CHEMBL214662)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C28H40O4/c1-16(13-26-17(2)18(3)27(31)32-26)23-10-11-24-20(7-6-12-28(23,24)5)8-9-21-14-22(29)15-25(30)19(21)4/h8-9,16-17,22-26,29-30H,3-4,6-7,10-15H2,1-2,5H3/b20-8+,21-9-/t16-,17+,22-,23-,24+,25+,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411178
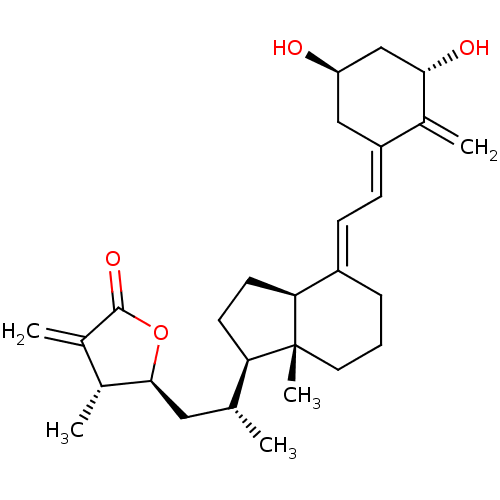
(CHEMBL410320)Show SMILES C[C@H](C[C@@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C28H40O4/c1-16(13-26-17(2)18(3)27(31)32-26)23-10-11-24-20(7-6-12-28(23,24)5)8-9-21-14-22(29)15-25(30)19(21)4/h8-9,16-17,22-26,29-30H,3-4,6-7,10-15H2,1-2,5H3/b20-8+,21-9-/t16-,17-,22-,23-,24+,25+,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411114
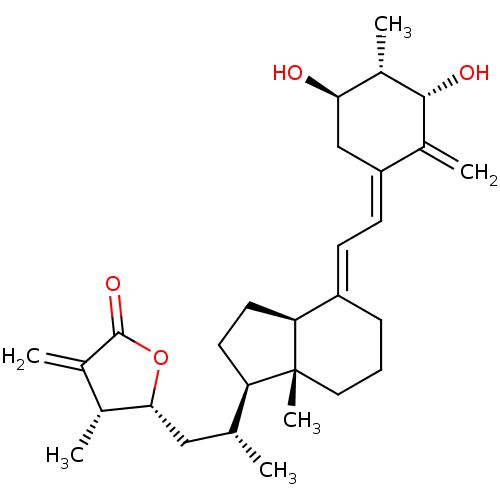
(CHEMBL213915)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)[C@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](C)[C@H](O)C1=C Show InChI InChI=1S/C29H42O4/c1-16(14-26-17(2)18(3)28(32)33-26)23-11-12-24-21(8-7-13-29(23,24)6)9-10-22-15-25(30)20(5)27(31)19(22)4/h9-10,16-17,20,23-27,30-31H,3-4,7-8,11-15H2,1-2,5-6H3/b21-9+,22-10-/t16-,17-,20+,23-,24+,25-,26-,27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411172

(CHEMBL214135)Show SMILES C[C@H](C[C@H]1OC(=O)C(=C)[C@@H]1C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)[C@H](C)[C@H](O)C1=C Show InChI InChI=1S/C29H42O4/c1-16(14-26-17(2)18(3)28(32)33-26)23-11-12-24-21(8-7-13-29(23,24)6)9-10-22-15-25(30)20(5)27(31)19(22)4/h9-10,16-17,20,23-27,30-31H,3-4,7-8,11-15H2,1-2,5-6H3/b21-9+,22-10-/t16-,17+,20+,23-,24+,25-,26-,27-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411151

(CHEMBL217265)Show SMILES CCCC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-6-7-10-25-21(4)30(34)35-29(25)16-19(2)26-13-14-27-22(9-8-15-31(26,27)5)11-12-23-17-24(32)18-28(33)20(23)3/h11-12,19,24-29,32-33H,3-4,6-10,13-18H2,1-2,5H3/b22-11+,23-12-/t19-,24-,25-,26-,27+,28+,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411168

(CHEMBL387286)Show SMILES CCCC[C@@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-6-7-10-25-21(4)30(34)35-29(25)16-19(2)26-13-14-27-22(9-8-15-31(26,27)5)11-12-23-17-24(32)18-28(33)20(23)3/h11-12,19,24-29,32-33H,3-4,6-10,13-18H2,1-2,5H3/b22-11+,23-12-/t19-,24-,25+,26-,27+,28+,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411107
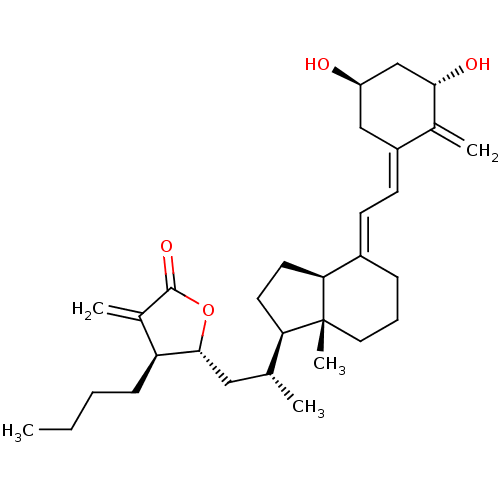
(CHEMBL214786)Show SMILES CCCC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-6-7-10-25-21(4)30(34)35-29(25)16-19(2)26-13-14-27-22(9-8-15-31(26,27)5)11-12-23-17-24(32)18-28(33)20(23)3/h11-12,19,24-29,32-33H,3-4,6-10,13-18H2,1-2,5H3/b22-11+,23-12-/t19-,24-,25+,26-,27+,28+,29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411181

(CHEMBL383856)Show SMILES CCCC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)C[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C31H46O4/c1-6-7-10-25-21(4)30(34)35-29(25)16-19(2)26-13-14-27-22(9-8-15-31(26,27)5)11-12-23-17-24(32)18-28(33)20(23)3/h11-12,19,24-29,32-33H,3-4,6-10,13-18H2,1-2,5H3/b22-11+,23-12-/t19-,24-,25-,26-,27+,28+,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411112

(CHEMBL385871)Show SMILES CC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](OCCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O6/c1-6-24-21(4)31(36)38-28(24)17-19(2)25-12-13-26-22(9-7-14-32(25,26)5)10-11-23-18-27(34)30(29(35)20(23)3)37-16-8-15-33/h10-11,19,24-30,33-35H,3-4,6-9,12-18H2,1-2,5H3/b22-10+,23-11-/t19-,24-,25-,26+,27-,28-,29-,30+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411109

(CHEMBL218183)Show SMILES CC[C@@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](OCCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O6/c1-6-24-21(4)31(36)38-28(24)17-19(2)25-12-13-26-22(9-7-14-32(25,26)5)10-11-23-18-27(34)30(29(35)20(23)3)37-16-8-15-33/h10-11,19,24-30,33-35H,3-4,6-9,12-18H2,1-2,5H3/b22-10+,23-11-/t19-,24+,25-,26+,27-,28-,29-,30+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411103

(CHEMBL217283)Show SMILES CC[C@H]1[C@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](CCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O5/c1-6-24-21(4)31(36)37-29(24)17-19(2)26-13-14-27-22(9-7-15-32(26,27)5)11-12-23-18-28(34)25(10-8-16-33)30(35)20(23)3/h11-12,19,24-30,33-35H,3-4,6-10,13-18H2,1-2,5H3/b22-11+,23-12-/t19-,24-,25+,26-,27+,28-,29+,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50411146

(CHEMBL385830)Show SMILES CC[C@H]1[C@@H](C[C@@H](C)[C@H]2CC[C@H]3\C(CCC[C@]23C)=C\C=C2\C[C@@H](O)[C@H](CCCO)[C@H](O)C2=C)OC(=O)C1=C Show InChI InChI=1S/C32H48O5/c1-6-24-21(4)31(36)37-29(24)17-19(2)26-13-14-27-22(9-7-15-32(26,27)5)11-12-23-18-28(34)25(10-8-16-33)30(35)20(23)3/h11-12,19,24-30,33-35H,3-4,6-10,13-18H2,1-2,5H3/b22-11+,23-12-/t19-,24-,25+,26-,27+,28-,29-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Teikyo University
Curated by ChEMBL
| Assay Description
Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method |
J Med Chem 49: 7063-75 (2006)
Article DOI: 10.1021/jm060797q
BindingDB Entry DOI: 10.7270/Q2F47QC9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data