

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202489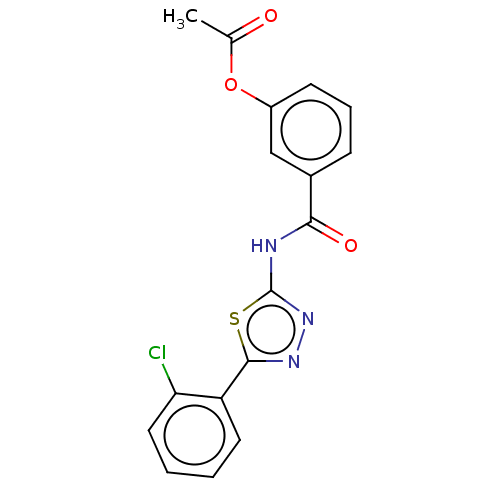 (CHEMBL3910200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202492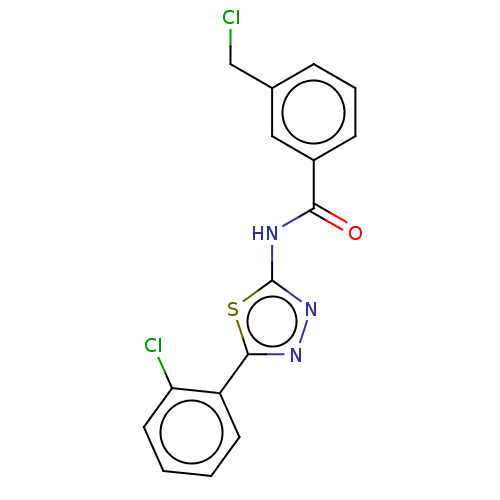 (CHEMBL3938124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202494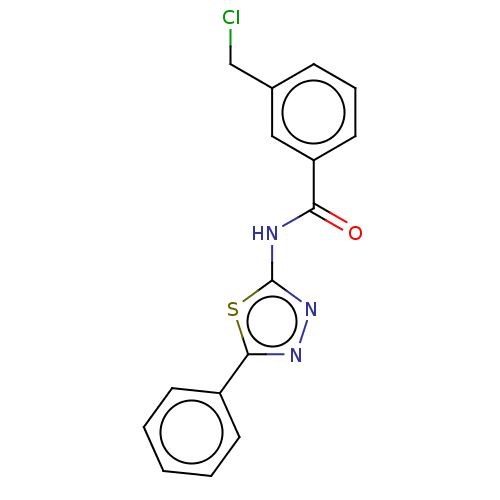 (CHEMBL3893245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202508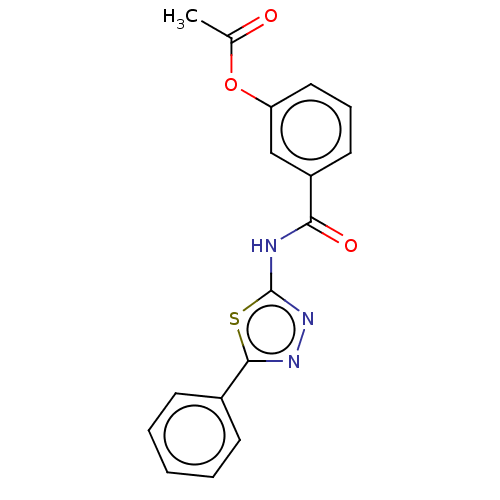 (CHEMBL3962271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202496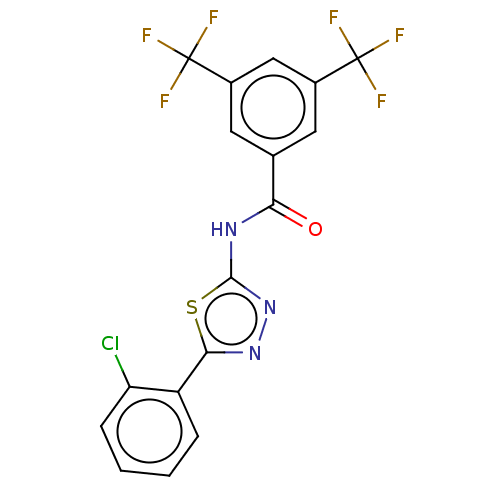 (CHEMBL3891487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202499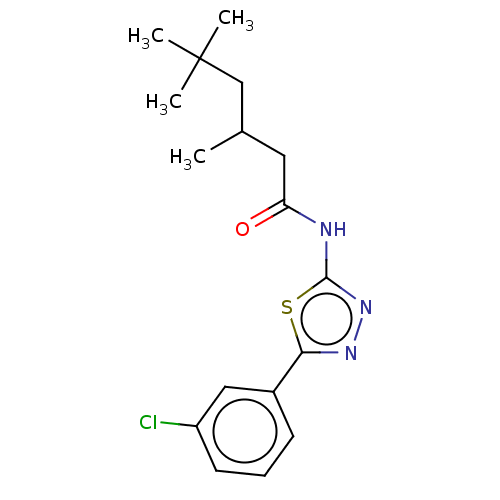 (CHEMBL3960527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202495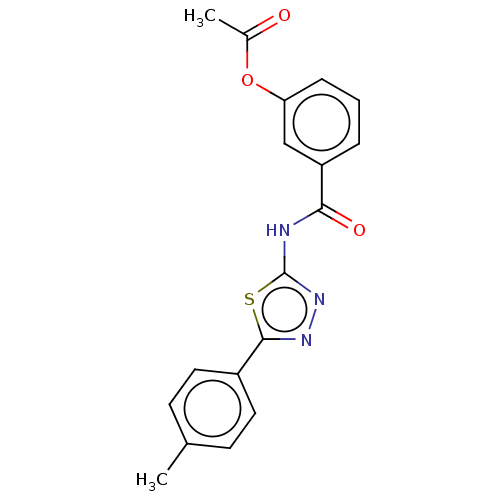 (CHEMBL3980699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202486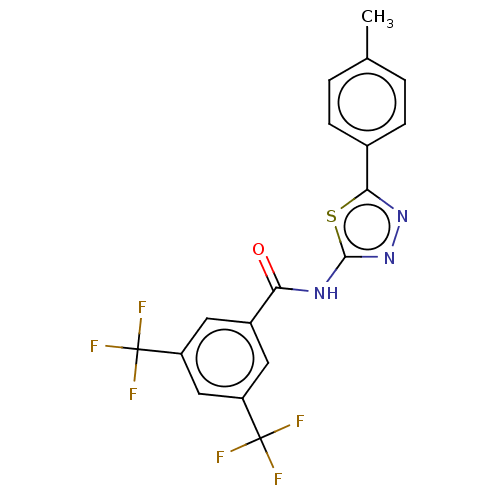 (CHEMBL3909539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202491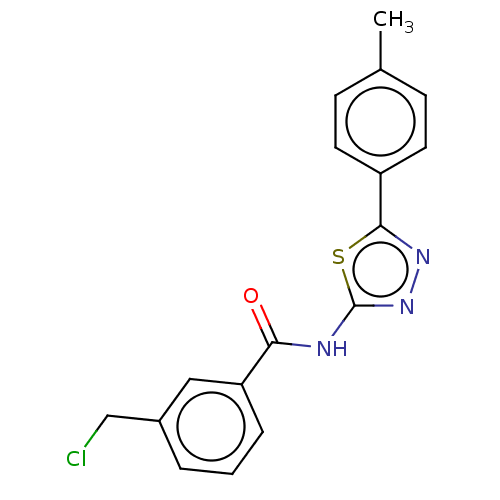 (CHEMBL3951830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202490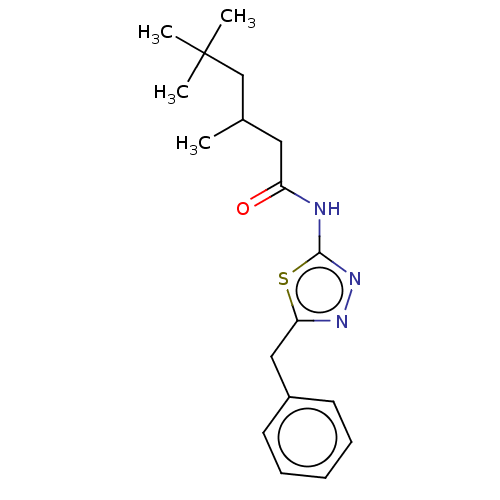 (CHEMBL3944788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202487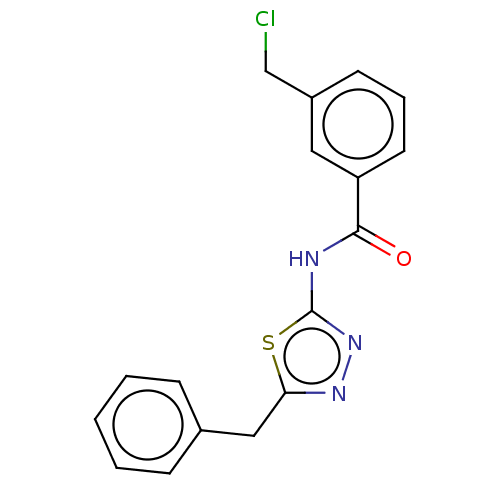 (CHEMBL3953512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202493 (CHEMBL3974229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202497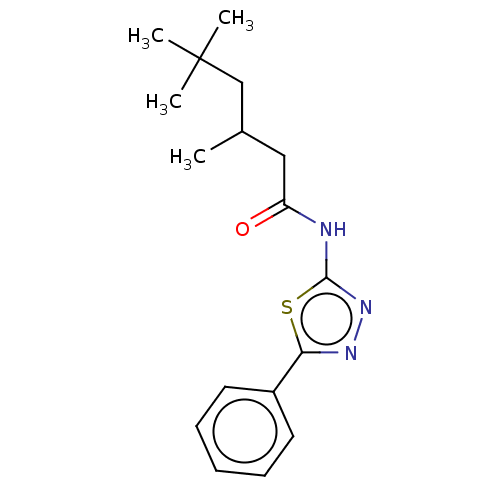 (CHEMBL3982229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202488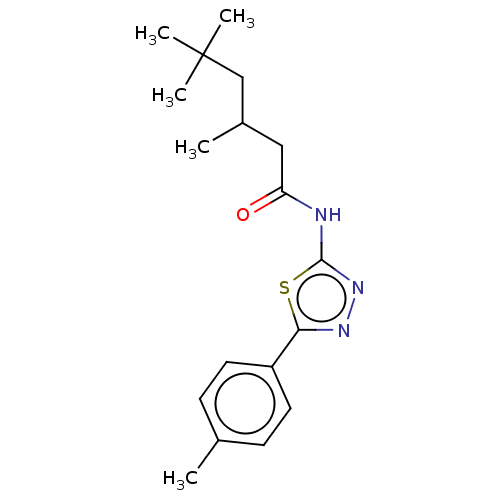 (CHEMBL3979735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured up to 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202496 (CHEMBL3891487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202492 (CHEMBL3938124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202489 (CHEMBL3910200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202508 (CHEMBL3962271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202494 (CHEMBL3893245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202486 (CHEMBL3909539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202495 (CHEMBL3980699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202493 (CHEMBL3974229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202491 (CHEMBL3951830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202499 (CHEMBL3960527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202487 (CHEMBL3953512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202497 (CHEMBL3982229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202488 (CHEMBL3979735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50202490 (CHEMBL3944788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of albino mouse brain AChE using acetylthiocholine iodide as substrate measured for 2 mins by Ellmans method | Eur J Med Chem 122: 557-573 (2016) Article DOI: 10.1016/j.ejmech.2016.06.046 BindingDB Entry DOI: 10.7270/Q23X88NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||