

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50571184 (CHEMBL4860610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571186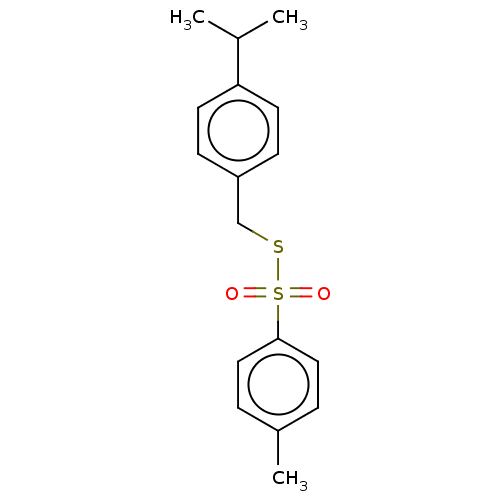 (CHEMBL4871975) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571186 (CHEMBL4871975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571187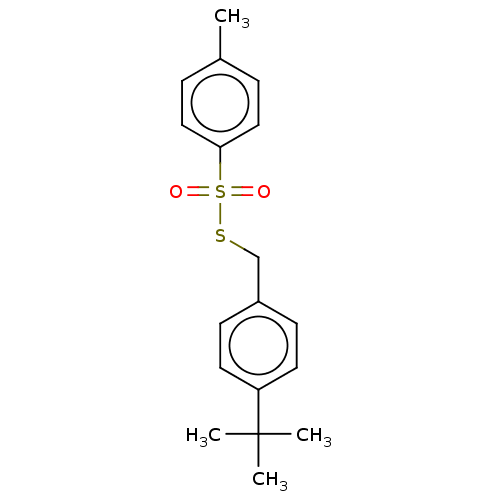 (CHEMBL4852721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571183 (CHEMBL4868918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571183 (CHEMBL4868918) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571187 (CHEMBL4852721) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571182 (CHEMBL4858117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571184 (CHEMBL4860610) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571180 (CHEMBL4863431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571182 (CHEMBL4858117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571179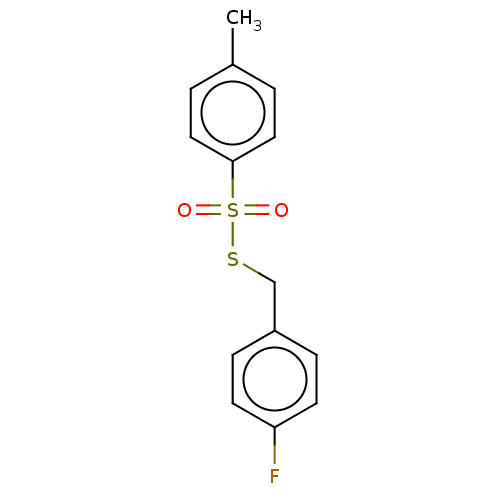 (CHEMBL4845755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571178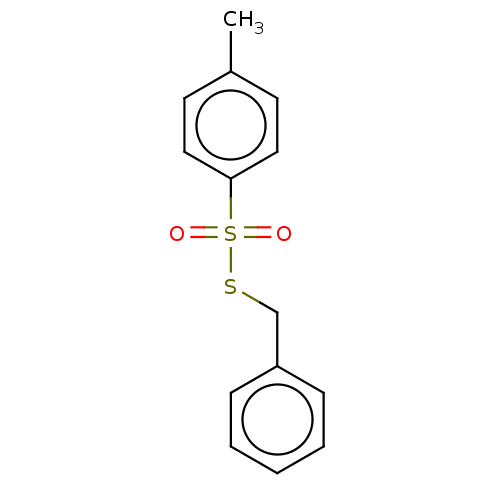 (CHEMBL1995010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571185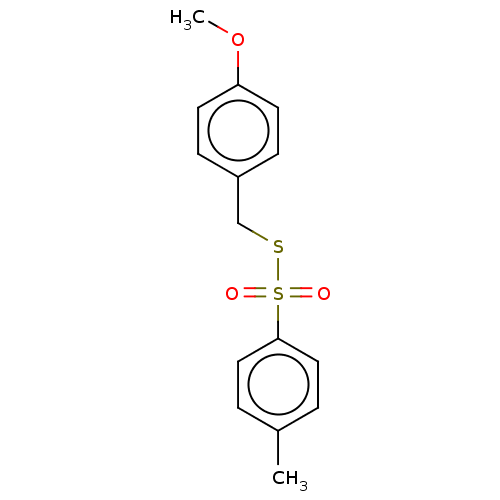 (CHEMBL4875944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571181 (CHEMBL4867081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571185 (CHEMBL4875944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571181 (CHEMBL4867081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571179 (CHEMBL4845755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571178 (CHEMBL1995010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571180 (CHEMBL4863431) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 714 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571186 (CHEMBL4871975) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571184 (CHEMBL4860610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571186 (CHEMBL4871975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571183 (CHEMBL4868918) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571183 (CHEMBL4868918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571184 (CHEMBL4860610) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571187 (CHEMBL4852721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571187 (CHEMBL4852721) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571182 (CHEMBL4858117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571185 (CHEMBL4875944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571182 (CHEMBL4858117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571181 (CHEMBL4867081) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571181 (CHEMBL4867081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571179 (CHEMBL4845755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571179 (CHEMBL4845755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571180 (CHEMBL4863431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571178 (CHEMBL1995010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571180 (CHEMBL4863431) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571185 (CHEMBL4875944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571178 (CHEMBL1995010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 10 mins followed by substrate addition ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116194 BindingDB Entry DOI: 10.7270/Q2154MTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||