 Found 1113 hits with Last Name = 'poso' and Initial = 'a'
Found 1113 hits with Last Name = 'poso' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50112086

(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human coagulation factor alpha-thrombin using Boc-Val-Pro-Arg-AMC as fluorogenic substrate measured at 1 min interval for 1 hr by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01635
BindingDB Entry DOI: 10.7270/Q2JM2F72 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597397
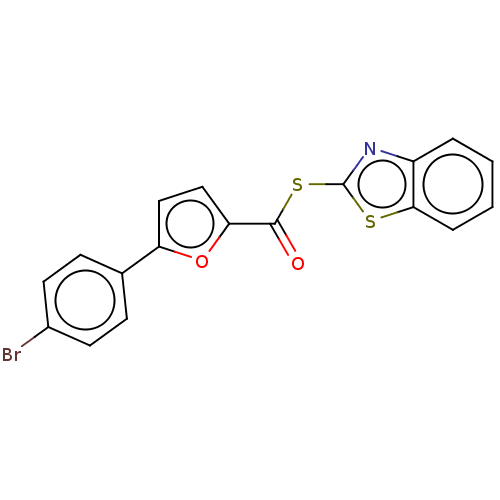
(CHEMBL5194755) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597385

(CHEMBL5169323) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597394

(CHEMBL5178465)Show SMILES COc1ccc2cc(ccc2c1)[C@H](C)C(=O)Sc1ncccn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597384

(CHEMBL5191017) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597386
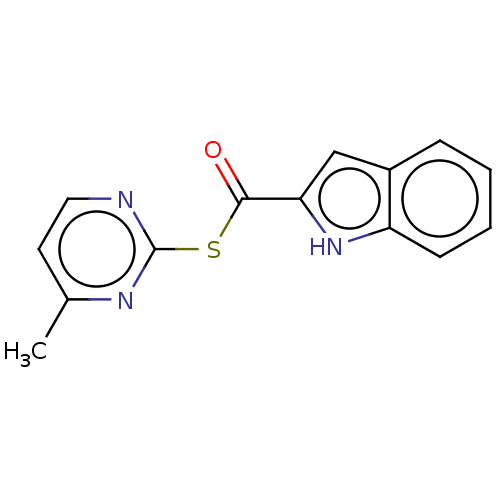
(CHEMBL5207081) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597382

(CHEMBL5207392) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597379

(CHEMBL5189863) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597399
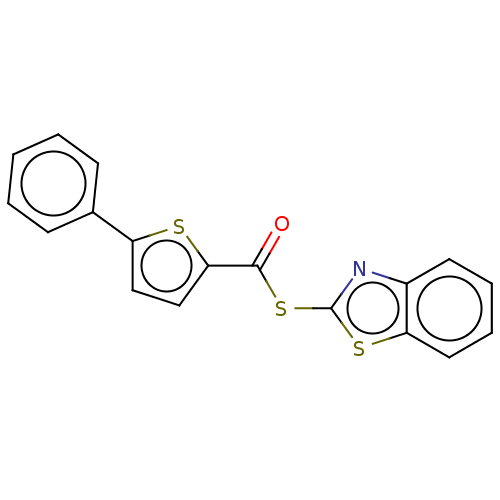
(CHEMBL5178568) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597375

(CHEMBL5204452) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597400
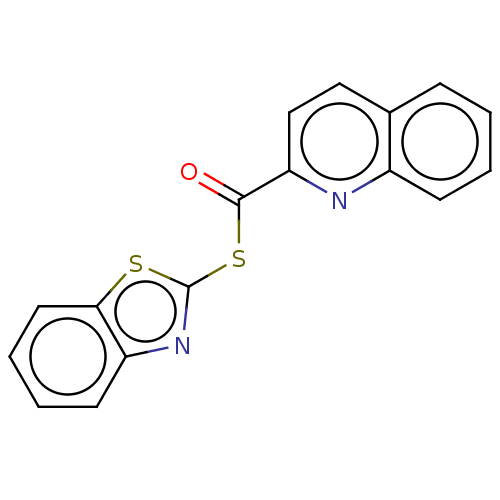
(CHEMBL5197891) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597395
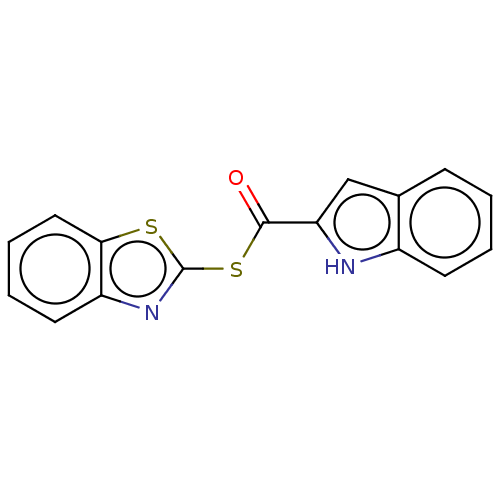
(CHEMBL5192654) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597368
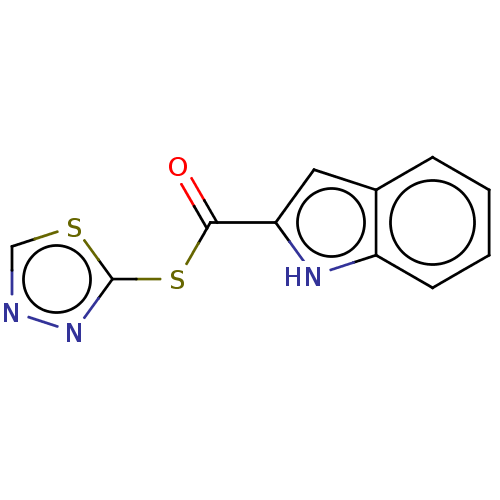
(CHEMBL5204130) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597378

(CHEMBL5202104)Show SMILES Cc1nnc(SC(=O)c2cc3ccccc3[nH]2)n1C(=O)c1cc2ccccc2[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597376

(CHEMBL5204064) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597377

(CHEMBL5207613) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597398
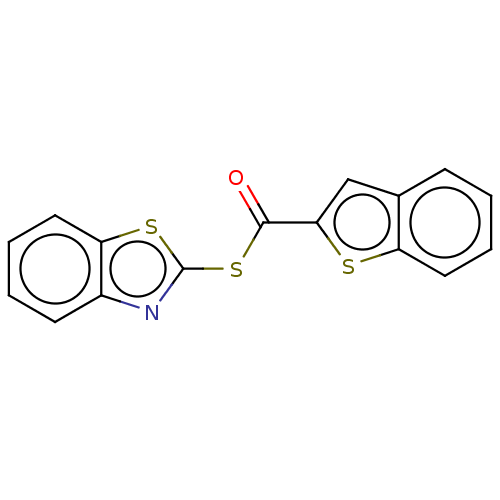
(CHEMBL5181137) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597372
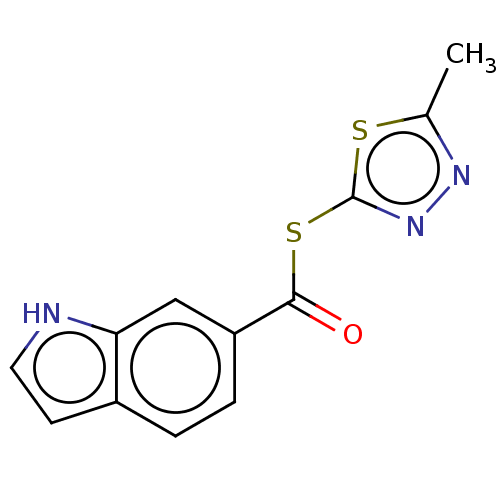
(CHEMBL5188939) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597373

(CHEMBL5199191) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597374
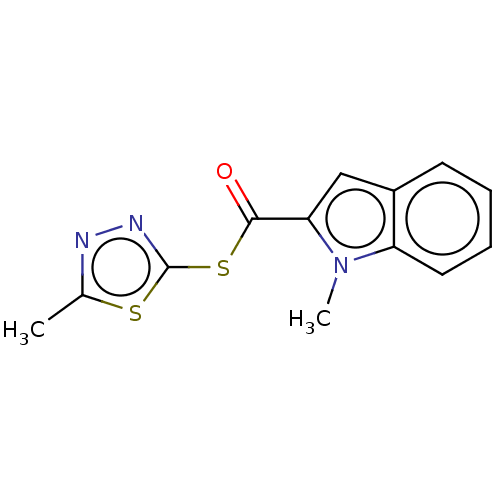
(CHEMBL5199531) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597371

(CHEMBL5173920) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597383

(CHEMBL5192027) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597396
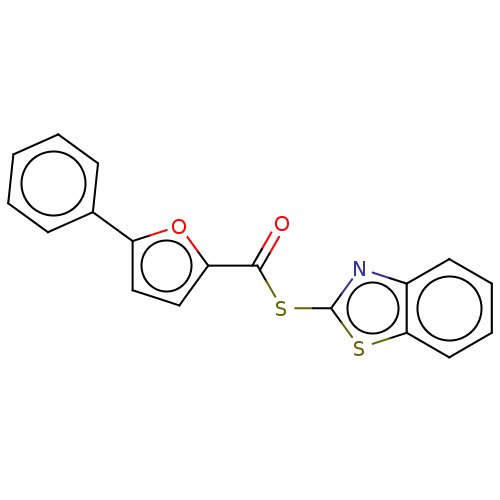
(CHEMBL5186708) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 612 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50597387
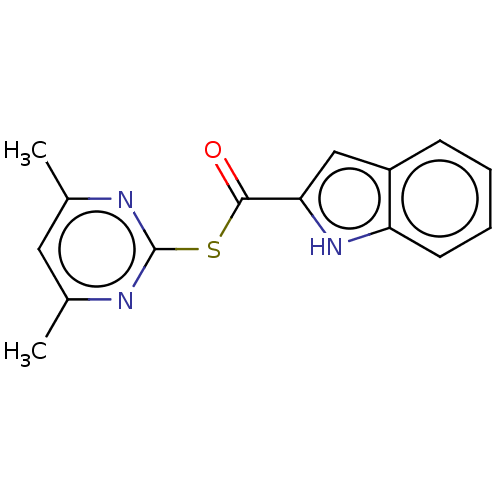
(CHEMBL5192248) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 773 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00636
BindingDB Entry DOI: 10.7270/Q29S1W2C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112086

(3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...)Show SMILES Cn1c(CNc2ccc(cc2)C(N)=N)nc2cc(ccc12)C(=O)N(CCC(O)=O)c1ccccn1 Show InChI InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human coagulation factor Xa using Boc-Ile-Glu-Gly-Arg-AMC as fluorogenic substrate measured at 1 min interval for 1 hr by fluorometric ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01635
BindingDB Entry DOI: 10.7270/Q2JM2F72 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50358746

(CHEMBL596015)Show InChI InChI=1S/C9H6OS/c10-5-7-6-11-9-4-2-1-3-8(7)9/h1-6H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of CYP2A6 in human liver microsomes assessed as coumarin 7-hydroxylation activity by double reciprocal plot analysis in prese... |
Bioorg Med Chem 19: 7186-93 (2011)
Article DOI: 10.1016/j.bmc.2011.09.054
BindingDB Entry DOI: 10.7270/Q25H7GPR |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50051495
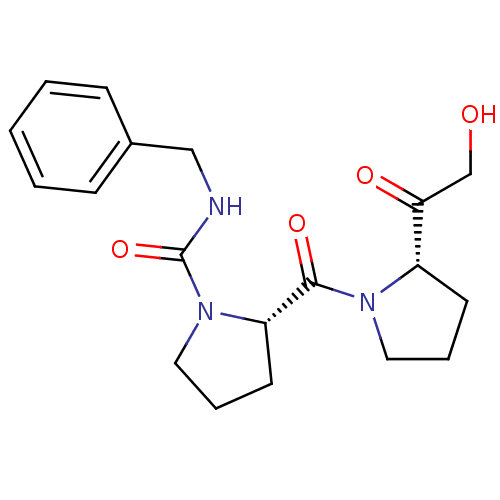
((S)-2-[(S)-2-(2-Hydroxy-acetyl)-pyrrolidine-1-carb...)Show SMILES OCC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)NCc1ccccc1 Show InChI InChI=1S/C19H25N3O4/c23-13-17(24)15-8-4-10-21(15)18(25)16-9-5-11-22(16)19(26)20-12-14-6-2-1-3-7-14/h1-3,6-7,15-16,23H,4-5,8-13H2,(H,20,26)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
Inhibition of pig brain POP |
J Med Chem 48: 7093-5 (2005)
Article DOI: 10.1021/jm0509187
BindingDB Entry DOI: 10.7270/Q2RR1XTB |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50051495

((S)-2-[(S)-2-(2-Hydroxy-acetyl)-pyrrolidine-1-carb...)Show SMILES OCC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)NCc1ccccc1 Show InChI InChI=1S/C19H25N3O4/c23-13-17(24)15-8-4-10-21(15)18(25)16-9-5-11-22(16)19(26)20-12-14-6-2-1-3-7-14/h1-3,6-7,15-16,23H,4-5,8-13H2,(H,20,26)/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50155838
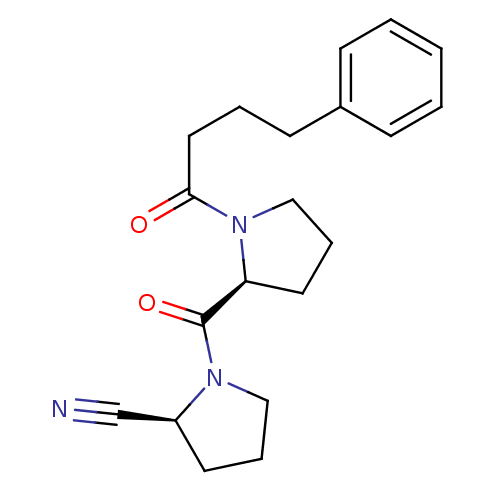
((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C20H25N3O2/c21-15-17-10-5-13-22(17)20(25)18-11-6-14-23(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170689
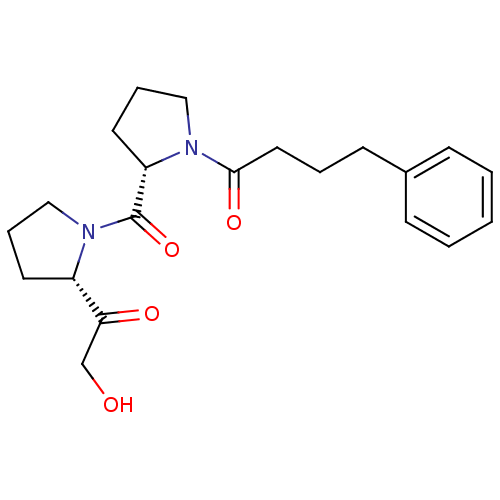
(1-{(S)-2-[(S)-2-(2-Hydroxy-acetyl)-pyrrolidine-1-c...)Show SMILES OCC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C21H28N2O4/c24-15-19(25)17-10-5-14-23(17)21(27)18-11-6-13-22(18)20(26)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,17-18,24H,4-6,9-15H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK2
(Homo sapiens (Human)) | BDBM50601538

(CHEMBL5090394)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3ncccc3F)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:29.31,wD:26.27,(3.85,-1.33,;3.08,,;1.54,,;.77,-1.33,;-.77,-1.33,;-1.54,,;-3.08,,;-3.85,1.33,;-5.39,1.33,;-6.16,2.67,;-7.7,2.67,;-8.47,1.33,;-7.7,,;-6.16,,;-5.39,-1.33,;-10.01,1.33,;-10.78,2.67,;-12.32,2.67,;-13.09,1.33,;-12.32,,;-10.78,,;-10.01,-1.33,;-3.08,2.67,;-3.85,4,;-1.54,2.67,;-.77,4,;.77,4,;1.54,2.67,;3.08,2.67,;3.85,4,;5.39,4,;3.08,5.33,;1.54,5.33,;-.77,1.33,;.77,1.33,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02144
BindingDB Entry DOI: 10.7270/Q2W66QTG |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50134170
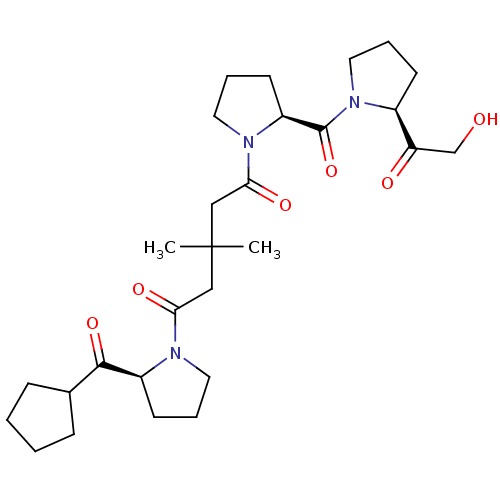
(1-((S)-2-Cyclopentanecarbonyl-pyrrolidin-1-yl)-5-{...)Show SMILES CC(C)(CC(=O)N1CCC[C@H]1C(=O)C1CCCC1)CC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)CO Show InChI InChI=1S/C28H43N3O6/c1-28(2,16-24(34)29-13-6-11-21(29)26(36)19-8-3-4-9-19)17-25(35)30-14-7-12-22(30)27(37)31-15-5-10-20(31)23(33)18-32/h19-22,32H,3-18H2,1-2H3/t20-,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50038879
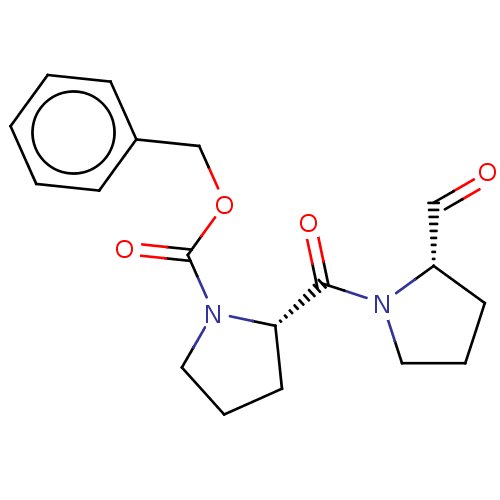
((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C18H22N2O4/c21-12-15-8-4-10-19(15)17(22)16-9-5-11-20(16)18(23)24-13-14-6-2-1-3-7-14/h1-3,6-7,12,15-16H,4-5,8-11,13H2/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170712
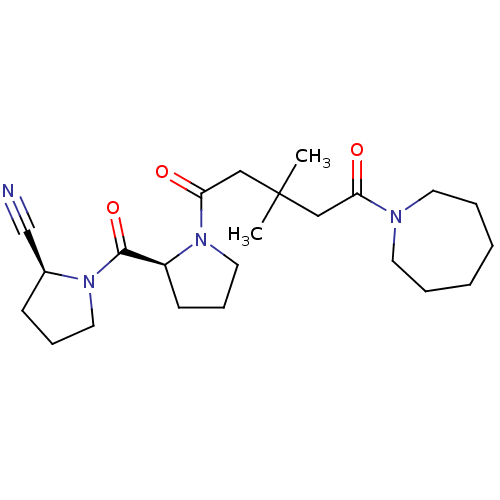
((S)-1-[(S)-1-(5-Azepan-1-yl-3,3-dimethyl-5-oxo-pen...)Show SMILES CC(C)(CC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N)CC(=O)N1CCCCCC1 Show InChI InChI=1S/C23H36N4O3/c1-23(2,15-20(28)25-11-5-3-4-6-12-25)16-21(29)27-14-8-10-19(27)22(30)26-13-7-9-18(26)17-24/h18-19H,3-16H2,1-2H3/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50134160
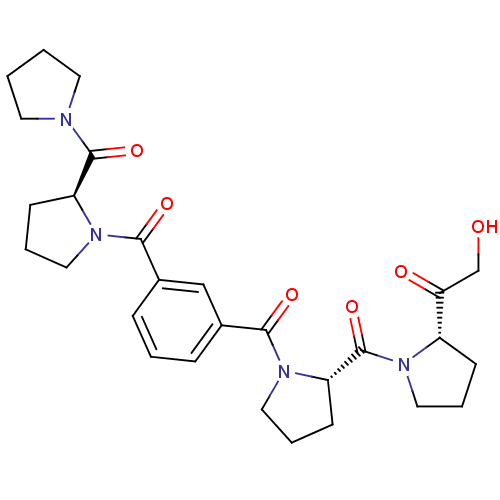
(2-Hydroxy-1-[(S)-1-((S)-1-{3-[(S)-2-(pyrrolidine-1...)Show SMILES OCC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)N1CCCC1 Show InChI InChI=1S/C28H36N4O6/c33-18-24(34)21-9-4-14-30(21)28(38)23-11-6-16-32(23)26(36)20-8-3-7-19(17-20)25(35)31-15-5-10-22(31)27(37)29-12-1-2-13-29/h3,7-8,17,21-23,33H,1-2,4-6,9-16,18H2/t21-,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50134150

((S)-1-{(S)-1-[5-((S)-2-Cyclopentanecarbonyl-pyrrol...)Show SMILES CC(C)(CC(=O)N1CCC[C@H]1C(=O)C1CCCC1)CC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C27H40N4O4/c1-27(2,16-23(32)30-14-6-11-21(30)25(34)19-8-3-4-9-19)17-24(33)31-15-7-12-22(31)26(35)29-13-5-10-20(29)18-28/h19-22H,3-17H2,1-2H3/t20-,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170693
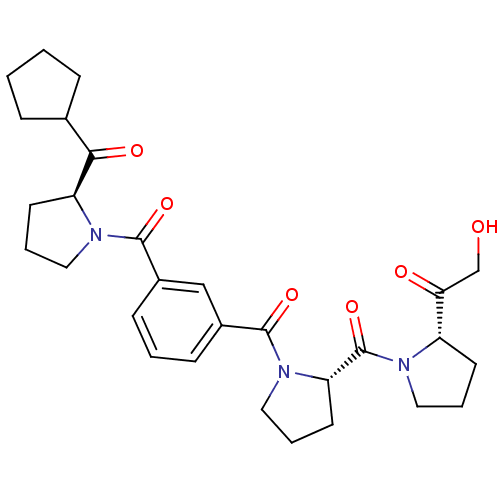
(1-((S)-1-{(S)-1-[3-((S)-2-Cyclopentanecarbonyl-pyr...)Show SMILES OCC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)C1CCCC1 Show InChI InChI=1S/C29H37N3O6/c33-18-25(34)22-11-4-14-30(22)29(38)24-13-6-16-32(24)28(37)21-10-3-9-20(17-21)27(36)31-15-5-12-23(31)26(35)19-7-1-2-8-19/h3,9-10,17,19,22-24,33H,1-2,4-8,11-16,18H2/t22-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308537
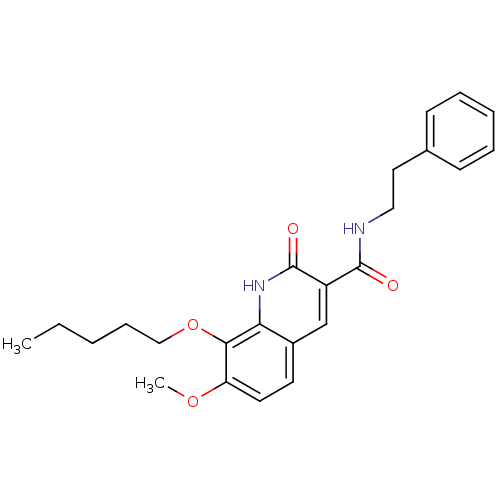
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H28N2O4/c1-3-4-8-15-30-22-20(29-2)12-11-18-16-19(24(28)26-21(18)22)23(27)25-14-13-17-9-6-5-7-10-17/h5-7,9-12,16H,3-4,8,13-15H2,1-2H3,(H,25,27)(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
Inhibition of [35S]GTP-gamma-S binding to human CB2 receptor expressed in CHO cells |
J Med Chem 49: 2022-7 (2006)
Article DOI: 10.1021/jm050879z
BindingDB Entry DOI: 10.7270/Q21J9C3R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
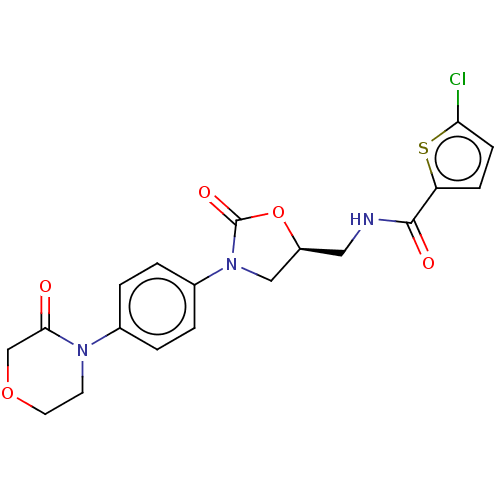
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human coagulation factor Xa using Boc-Ile-Glu-Gly-Arg-AMC as fluorogenic substrate measured at 1 min interval for 1 hr by fluorometric ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01635
BindingDB Entry DOI: 10.7270/Q2JM2F72 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170686

((S)-1-{(S)-1-[3-((S)-2-Cyclohexanecarbonyl-pyrroli...)Show SMILES O=C([C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N)C1CCCCC1 Show InChI InChI=1S/C29H36N4O4/c30-19-23-12-5-15-31(23)29(37)25-14-7-17-33(25)28(36)22-11-4-10-21(18-22)27(35)32-16-6-13-24(32)26(34)20-8-2-1-3-9-20/h4,10-11,18,20,23-25H,1-3,5-9,12-17H2/t23-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170705
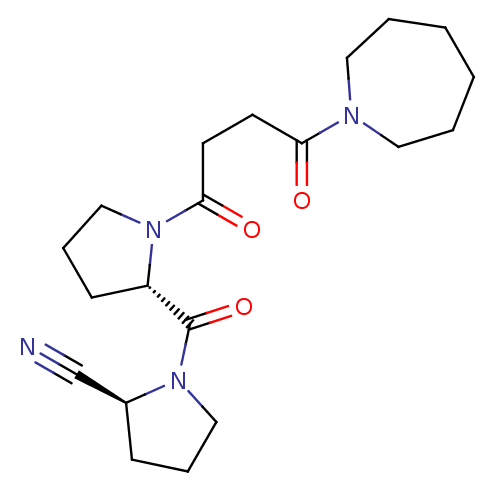
((S)-1-[(S)-1-(4-Azepan-1-yl-4-oxo-butyryl)-pyrroli...)Show SMILES O=C(CCC(=O)N1CCCCCC1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C20H30N4O3/c21-15-16-7-5-13-23(16)20(27)17-8-6-14-24(17)19(26)10-9-18(25)22-11-3-1-2-4-12-22/h16-17H,1-14H2/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308539
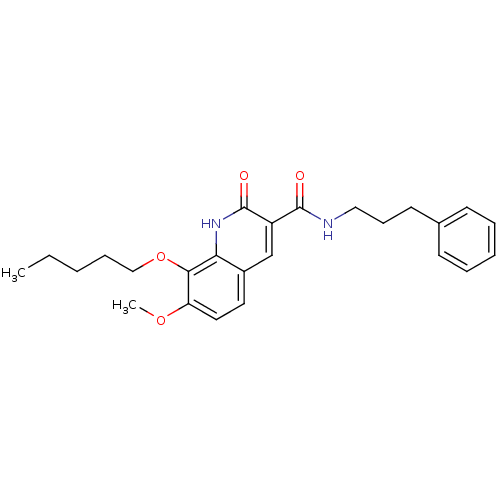
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C25H30N2O4/c1-3-4-8-16-31-23-21(30-2)14-13-19-17-20(25(29)27-22(19)23)24(28)26-15-9-12-18-10-6-5-7-11-18/h5-7,10-11,13-14,17H,3-4,8-9,12,15-16H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
Inhibition of [35S]GTP-gamma-S binding to human CB2 receptor expressed in CHO cells |
J Med Chem 49: 2022-7 (2006)
Article DOI: 10.1021/jm050879z
BindingDB Entry DOI: 10.7270/Q21J9C3R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50176777
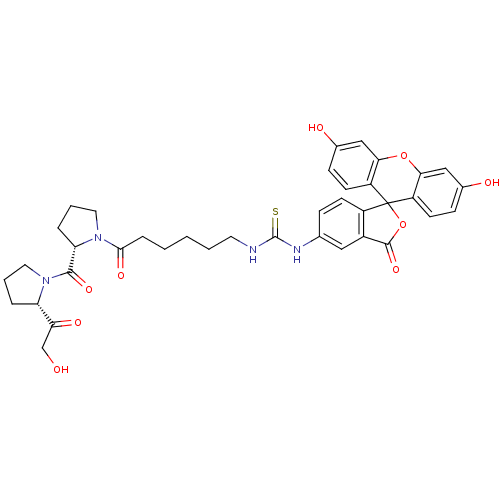
(4-fluoresceinthiocarbamoyl-6-aminocaproyl-L-prolyl...)Show SMILES OCC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCCCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12 |r| Show InChI InChI=1S/C38H40N4O9S/c43-21-31(46)29-6-4-17-42(29)35(48)30-7-5-16-41(30)34(47)8-2-1-3-15-39-37(52)40-22-9-12-26-25(18-22)36(49)51-38(26)27-13-10-23(44)19-32(27)50-33-20-24(45)11-14-28(33)38/h9-14,18-20,29-30,43-45H,1-8,15-17,21H2,(H2,39,40,52)/t29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
Inhibition of pig brain POP |
J Med Chem 48: 7093-5 (2005)
Article DOI: 10.1021/jm0509187
BindingDB Entry DOI: 10.7270/Q2RR1XTB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK2
(Homo sapiens (Human)) | BDBM50601534
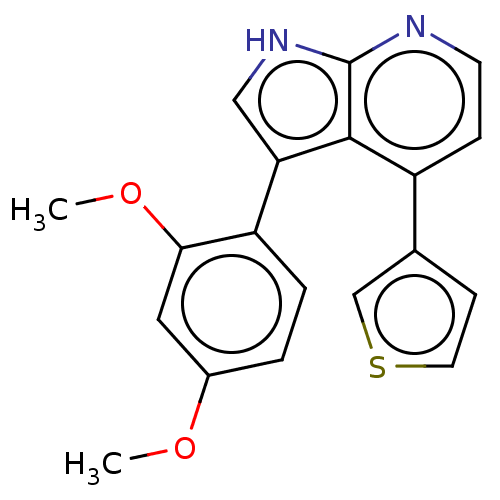
(CHEMBL4539742)Show SMILES COc1ccc(-c2c[nH]c3nccc(-c4ccsc4)c23)c(OC)c1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02144
BindingDB Entry DOI: 10.7270/Q2W66QTG |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170682
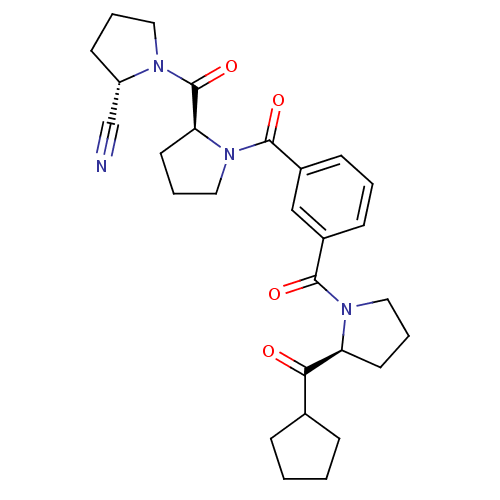
((S)-1-((S)-1-(3-((S)-2-(cyclopentanecarbonyl)pyrro...)Show SMILES O=C(C1CCCC1)[C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C28H34N4O4/c29-18-22-11-4-14-30(22)28(36)24-13-6-16-32(24)27(35)21-10-3-9-20(17-21)26(34)31-15-5-12-23(31)25(33)19-7-1-2-8-19/h3,9-10,17,19,22-24H,1-2,4-8,11-16H2/t22-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170707
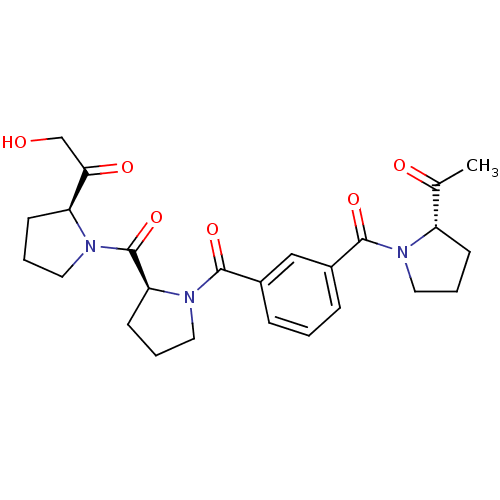
(1-((S)-1-{(S)-1-[3-((S)-2-Acetyl-pyrrolidine-1-car...)Show SMILES CC(=O)[C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)CO Show InChI InChI=1S/C25H31N3O6/c1-16(30)19-8-3-11-26(19)23(32)17-6-2-7-18(14-17)24(33)28-13-5-10-21(28)25(34)27-12-4-9-20(27)22(31)15-29/h2,6-7,14,19-21,29H,3-5,8-13,15H2,1H3/t19-,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170683
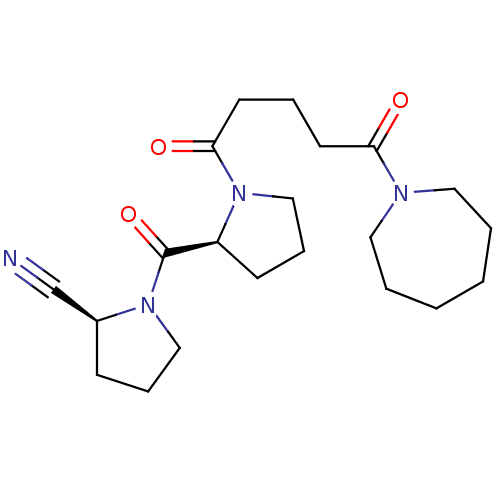
((S)-1-[(S)-1-(5-Azepan-1-yl-5-oxo-pentanoyl)-pyrro...)Show SMILES O=C(CCCC(=O)N1CCCCCC1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C21H32N4O3/c22-16-17-8-6-14-24(17)21(28)18-9-7-15-25(18)20(27)11-5-10-19(26)23-12-3-1-2-4-13-23/h17-18H,1-15H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50298655
(3-(4,5-Dihydrooxazol-2-yl)phenyl cyclohexylcarbama...)Show InChI InChI=1S/C16H20N2O3/c19-16(18-13-6-2-1-3-7-13)21-14-8-4-5-12(11-14)15-17-9-10-20-15/h4-5,8,11,13H,1-3,6-7,9-10H2,(H,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Helsinki University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Wistar rat brain assessed as by liquid scintillation counting |
Eur J Med Chem 44: 4179-91 (2009)
Article DOI: 10.1016/j.ejmech.2009.05.012
BindingDB Entry DOI: 10.7270/Q2FT8M3R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50170692
((S)-1-{(S)-1-[3-((S)-2-Benzoyl-pyrrolidine-1-carbo...)Show SMILES O=C([C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)c1ccccc1)N1CCC[C@H]1C#N Show InChI InChI=1S/C29H30N4O4/c30-19-23-12-5-15-31(23)29(37)25-14-7-17-33(25)28(36)22-11-4-10-21(18-22)27(35)32-16-6-13-24(32)26(34)20-8-2-1-3-9-20/h1-4,8-11,18,23-25H,5-7,12-17H2/t23-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50134167

((S)-1-((S)-1-{3-[(S)-2-(Pyrrolidine-1-carbonyl)-py...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)N1CCCC1 Show InChI InChI=1S/C27H34N4O5/c32-18-21-9-4-14-29(21)27(36)23-11-6-16-31(23)25(34)20-8-3-7-19(17-20)24(33)30-15-5-10-22(30)26(35)28-12-1-2-13-28/h3,7-8,17-18,21-23H,1-2,4-6,9-16H2/t21-,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against porcine prolyl oligopeptidase by using 4 mM Suc-Gly-Pro-7-amido-4-methylcoumarin as substrate (pH 7.0) at 30 deg... |
J Med Chem 48: 4772-82 (2005)
Article DOI: 10.1021/jm0500020
BindingDB Entry DOI: 10.7270/Q22B8ZT2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data