 Found 13 hits with Last Name = 'sivakumar' and Initial = 'a'
Found 13 hits with Last Name = 'sivakumar' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279131

(CHEMBL4174467)Show SMILES OC(=O)CN1C(=O)\C(=N/C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C22H17N3O4S/c26-18-10-14-6-2-1-5-13(14)9-15(18)11-23-22(30)24-20-16-7-3-4-8-17(16)25(21(20)29)12-19(27)28/h1-10,26H,11-12H2,(H,23,30)(H,27,28)/b24-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition over 48 hr of BAL strain HIV infrction of HeLa Magi cells expressing CCR5 |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279113
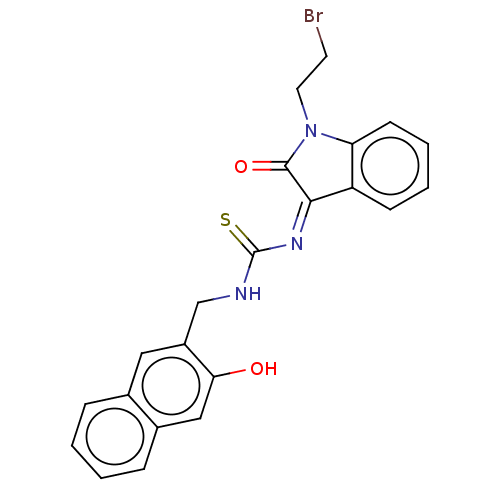
(CHEMBL4160421)Show SMILES Oc1cc2ccccc2cc1CNC(=S)\N=C1/C(=O)N(CCBr)c2ccccc12 Show InChI InChI=1S/C22H18BrN3O2S/c23-9-10-26-18-8-4-3-7-17(18)20(21(26)28)25-22(29)24-13-16-11-14-5-1-2-6-15(14)12-19(16)27/h1-8,11-12,27H,9-10,13H2,(H,24,29)/b25-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Antagonistic activity against the P2X7 ion channel |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279127
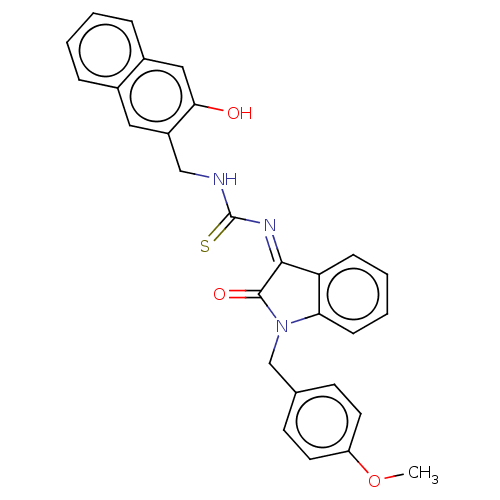
(CHEMBL4174072)Show SMILES COc1ccc(CN2C(=O)\C(=N/C(=S)NCc3cc4ccccc4cc3O)c3ccccc23)cc1 Show InChI InChI=1S/C28H23N3O3S/c1-34-22-12-10-18(11-13-22)17-31-24-9-5-4-8-23(24)26(27(31)33)30-28(35)29-16-21-14-19-6-2-3-7-20(19)15-25(21)32/h2-15,32H,16-17H2,1H3,(H,29,35)/b30-26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279128
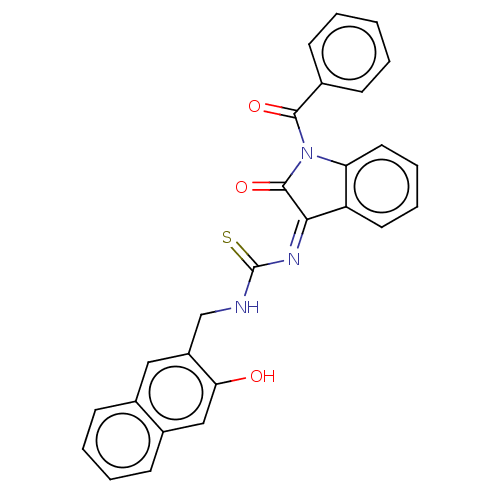
(CHEMBL4163792)Show SMILES Oc1cc2ccccc2cc1CNC(=S)\N=C1/C(=O)N(C(=O)c2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H19N3O3S/c31-23-15-19-11-5-4-10-18(19)14-20(23)16-28-27(34)29-24-21-12-6-7-13-22(21)30(26(24)33)25(32)17-8-2-1-3-9-17/h1-15,31H,16H2,(H,28,34)/b29-24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled nonpeptide from purified recombinant human alphaV-beta3 integrin |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279117
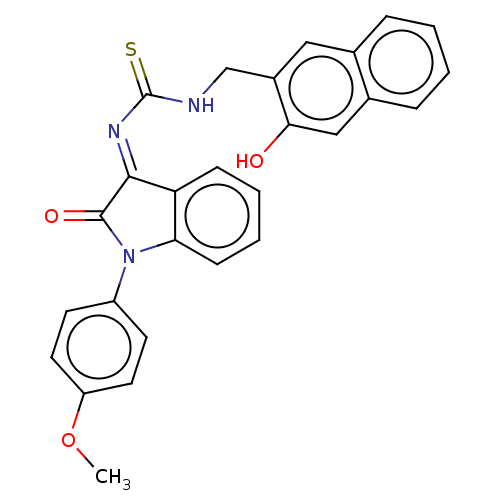
(CHEMBL4175518)Show SMILES COc1ccc(cc1)N1C(=O)\C(=N\C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C27H21N3O3S/c1-33-21-12-10-20(11-13-21)30-23-9-5-4-8-22(23)25(26(30)32)29-27(34)28-16-19-14-17-6-2-3-7-18(17)15-24(19)31/h2-15,31H,16H2,1H3,(H,28,34)/b29-25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279116

(CHEMBL4167651)Show SMILES CC(=O)N1C(=O)\C(=N/C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C22H17N3O3S/c1-13(26)25-18-9-5-4-8-17(18)20(21(25)28)24-22(29)23-12-16-10-14-6-2-3-7-15(14)11-19(16)27/h2-11,27H,12H2,1H3,(H,23,29)/b24-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279115

(CHEMBL4171024)Show SMILES CCCN1C(=O)\C(=N/C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C23H21N3O2S/c1-2-11-26-19-10-6-5-9-18(19)21(22(26)28)25-23(29)24-14-17-12-15-7-3-4-8-16(15)13-20(17)27/h3-10,12-13,27H,2,11,14H2,1H3,(H,24,29)/b25-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Antagonistic activity against the P2X7 ion channel |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279112
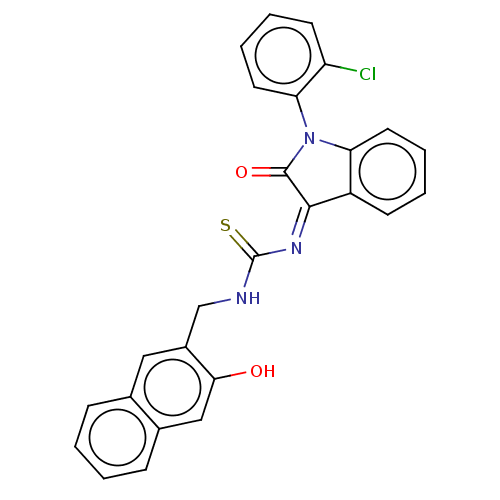
(CHEMBL4163798)Show SMILES Oc1cc2ccccc2cc1CNC(=S)\N=C1/C(=O)N(c2ccccc12)c1ccccc1Cl Show InChI InChI=1S/C26H18ClN3O2S/c27-20-10-4-6-12-22(20)30-21-11-5-3-9-19(21)24(25(30)32)29-26(33)28-15-18-13-16-7-1-2-8-17(16)14-23(18)31/h1-14,31H,15H2,(H,28,33)/b29-24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Antagonistic activity against the P2X7 ion channel |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279114

(CHEMBL4167252)Show SMILES Oc1cc2ccccc2cc1CNC(=S)\N=C1/C(=O)N(c2ccccc12)c1ccccc1 Show InChI InChI=1S/C26H19N3O2S/c30-23-15-18-9-5-4-8-17(18)14-19(23)16-27-26(32)28-24-21-12-6-7-13-22(21)29(25(24)31)20-10-2-1-3-11-20/h1-15,30H,16H2,(H,27,32)/b28-24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Antagonistic activity against the P2X7 ion channel |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279129

(CHEMBL4163389)Show SMILES CN1C(=O)\C(=N/C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C21H17N3O2S/c1-24-17-9-5-4-8-16(17)19(20(24)26)23-21(27)22-12-15-10-13-6-2-3-7-14(13)11-18(15)25/h2-11,25H,12H2,1H3,(H,22,27)/b23-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279118
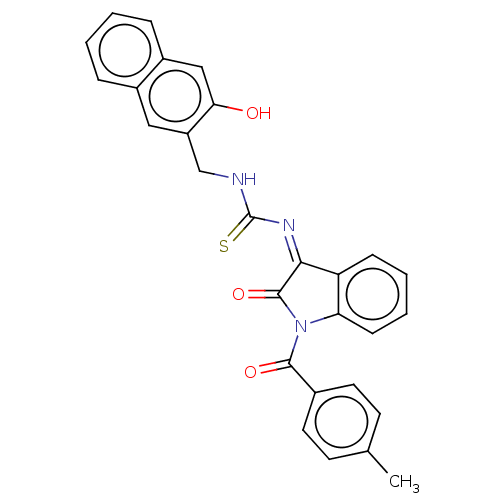
(CHEMBL4166152)Show SMILES Cc1ccc(cc1)C(=O)N1C(=O)\C(=N/C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C28H21N3O3S/c1-17-10-12-18(13-11-17)26(33)31-23-9-5-4-8-22(23)25(27(31)34)30-28(35)29-16-21-14-19-6-2-3-7-20(19)15-24(21)32/h2-15,32H,16H2,1H3,(H,29,35)/b30-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279130

(CHEMBL4159339)Show SMILES CC(=O)CN1C(=O)\C(=N/C(=S)NCc2cc3ccccc3cc2O)c2ccccc12 Show InChI InChI=1S/C23H19N3O3S/c1-14(27)13-26-19-9-5-4-8-18(19)21(22(26)29)25-23(30)24-12-17-10-15-6-2-3-7-16(15)11-20(17)28/h2-11,28H,12-13H2,1H3,(H,24,30)/b25-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric method |
Eur J Med Chem 141: 417-426 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.076
BindingDB Entry DOI: 10.7270/Q26112TS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data