 Found 103 hits with Last Name = 'viacava follis' and Initial = 'a'
Found 103 hits with Last Name = 'viacava follis' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571038
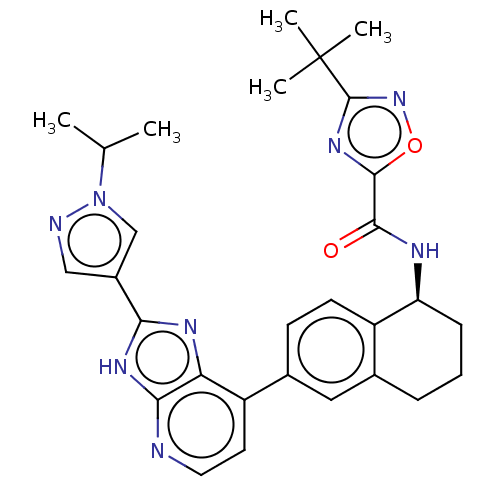
(CHEMBL4868813)Show SMILES CC(C)n1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514956
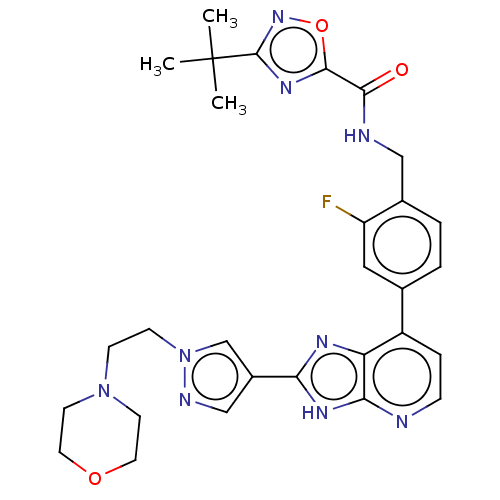
(US11098041, Example 187)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)-c1cnn(CCN2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514935
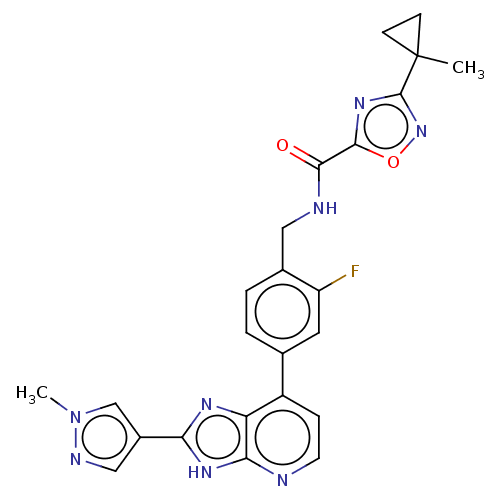
(US11098041, Example 165)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C2(C)CC2)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571039
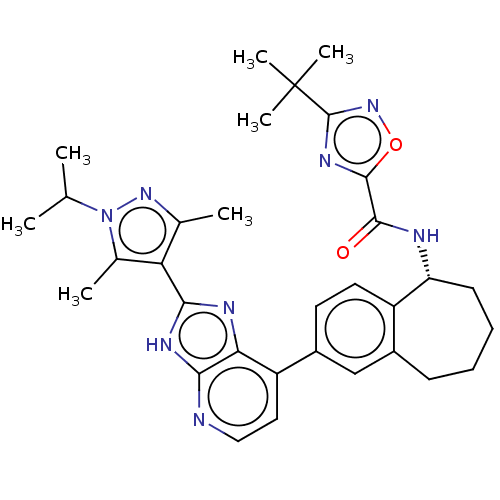
(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514891
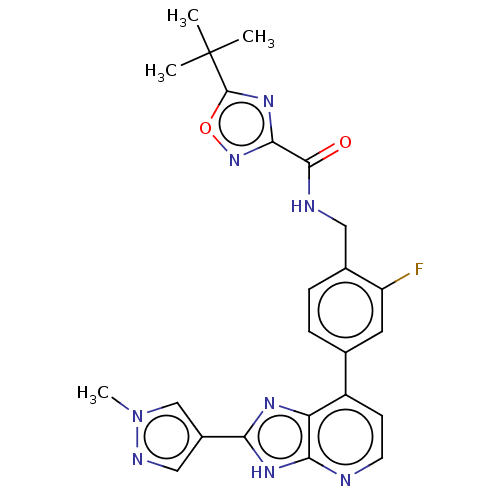
(US11098041, Example 116)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2noc(n2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514887
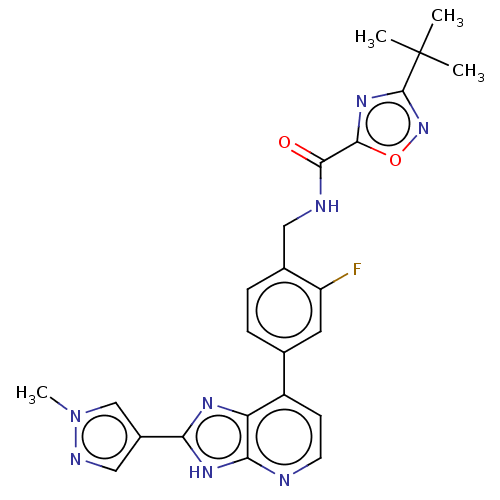
(US11098041, Example 112)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514915

(US11098041, Example 144)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2cc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514904
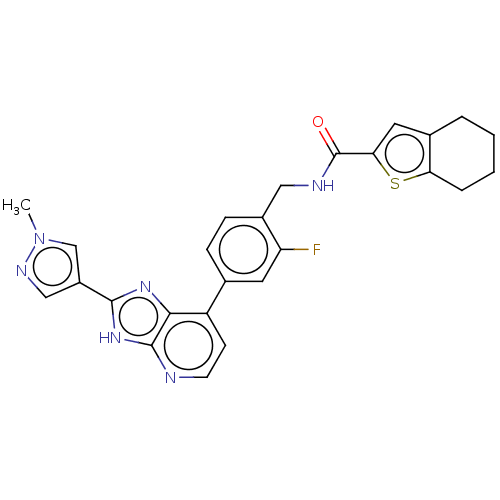
(US11098041, Example 132)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2cc3CCCCc3s2)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571033
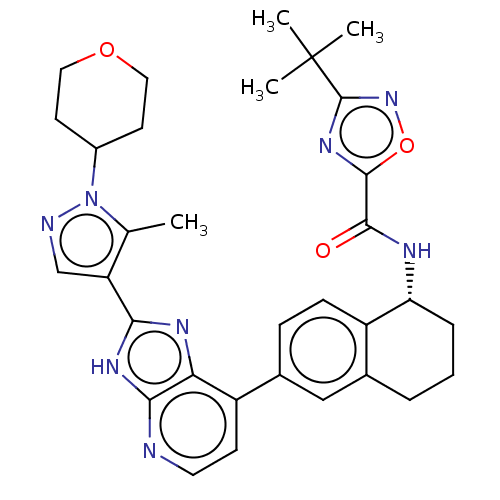
(CHEMBL4876326)Show SMILES Cc1c(cnn1C1CCOCC1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571037
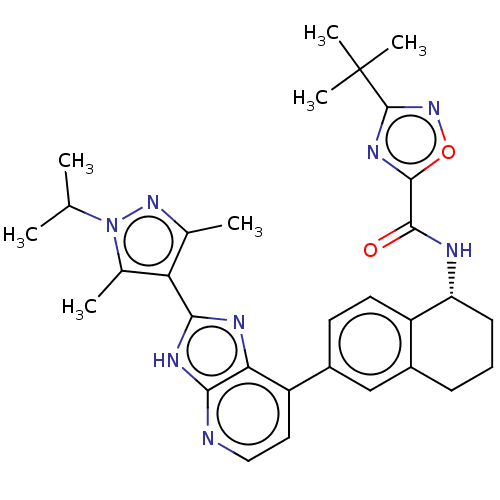
(CHEMBL4862532)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571029
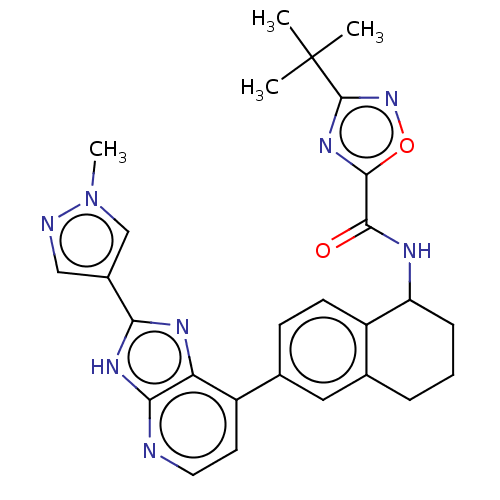
(CHEMBL4855860)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2C(CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514897
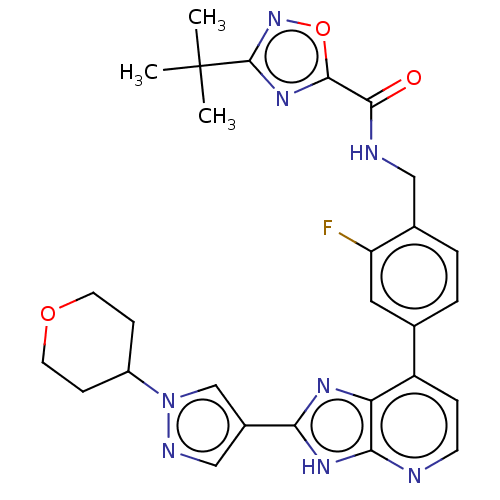
(US11098041, Example 124)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)-c1cnn(c1)C1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466204
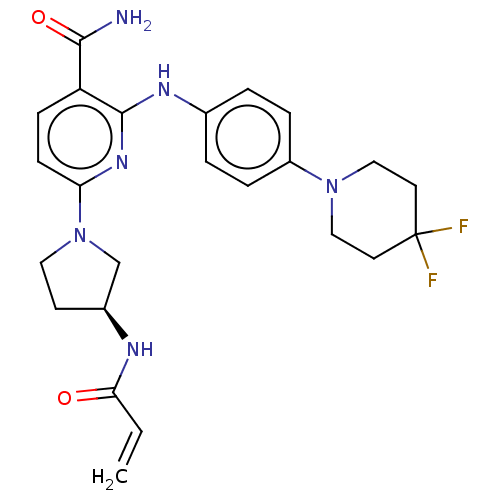
(CHEMBL4288997)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(cc1)N1CCC(F)(F)CC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C24H28F2N6O2/c1-2-21(33)28-17-9-12-32(15-17)20-8-7-19(22(27)34)23(30-20)29-16-3-5-18(6-4-16)31-13-10-24(25,26)11-14-31/h2-8,17H,1,9-15H2,(H2,27,34)(H,28,33)(H,29,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571034
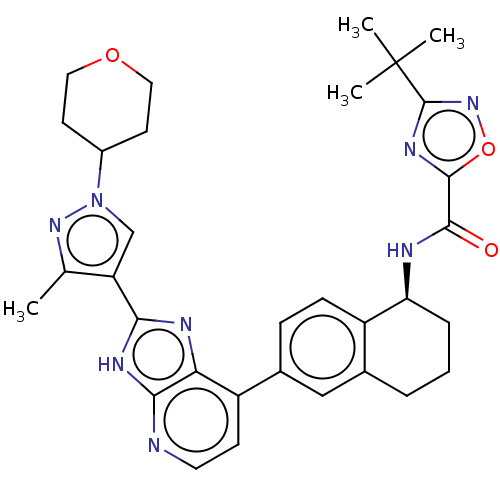
(CHEMBL4872092)Show SMILES Cc1nn(cc1-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C)C1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388189
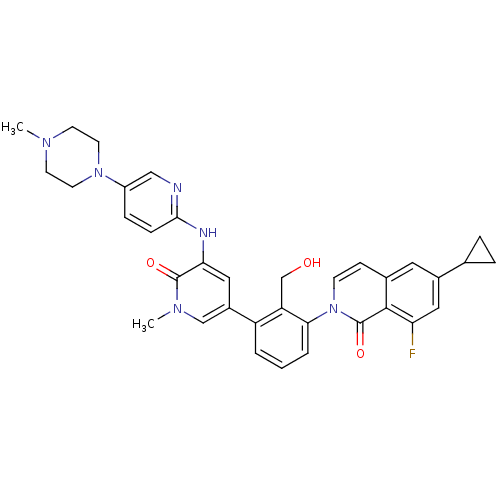
(CHEMBL2057918)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)nc1 Show InChI InChI=1S/C35H35FN6O3/c1-39-12-14-41(15-13-39)26-8-9-32(37-19-26)38-30-18-25(20-40(2)34(30)44)27-4-3-5-31(28(27)21-43)42-11-10-23-16-24(22-6-7-22)17-29(36)33(23)35(42)45/h3-5,8-11,16-20,22,43H,6-7,12-15,21H2,1-2H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514831
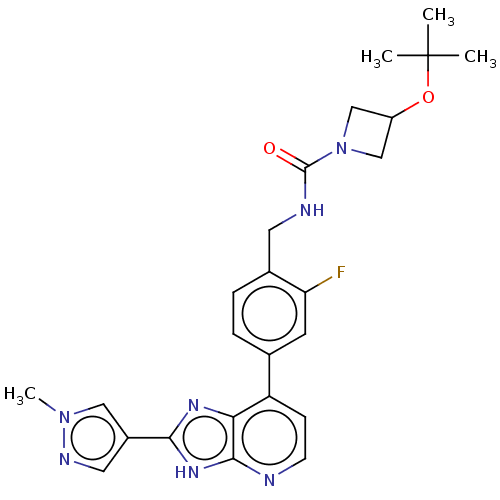
(US11098041, Example 55)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)N2CC(C2)OC(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571035
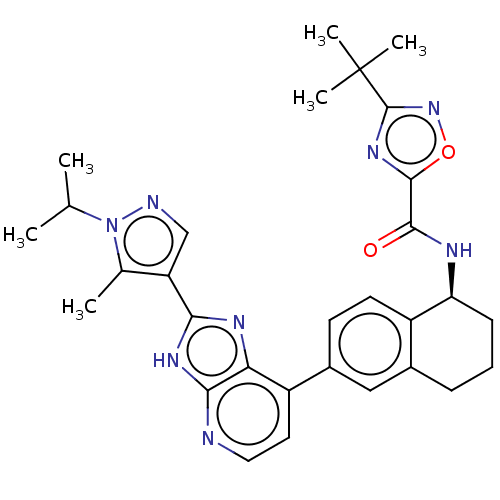
(CHEMBL4857459)Show SMILES CC(C)n1ncc(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571036
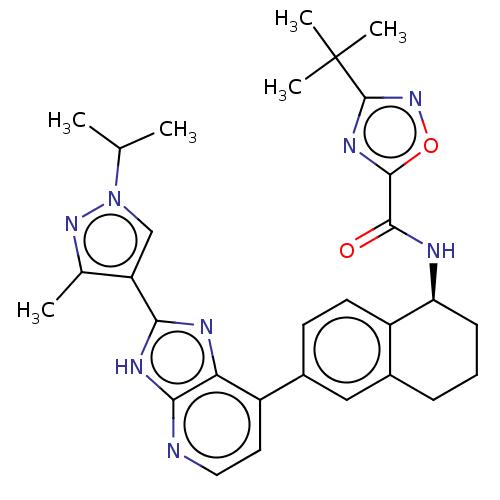
(CHEMBL4851951)Show SMILES CC(C)n1cc(c(C)n1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571031
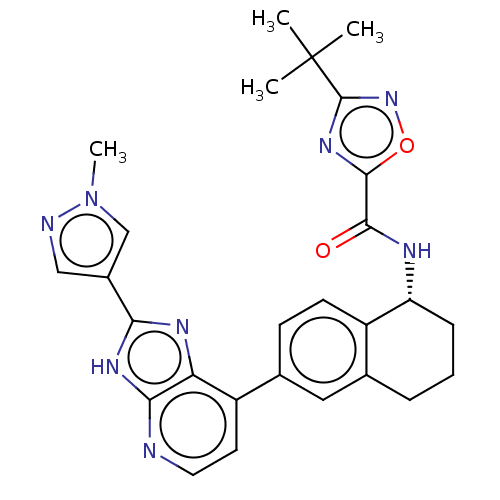
(CHEMBL4859888)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571032
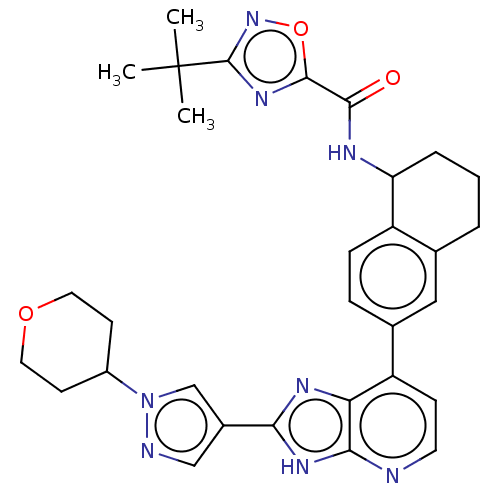
(CHEMBL4848010)Show SMILES CC(C)(C)c1noc(n1)C(=O)NC1CCCc2cc(ccc12)-c1ccnc2[nH]c(nc12)-c1cnn(c1)C1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514929
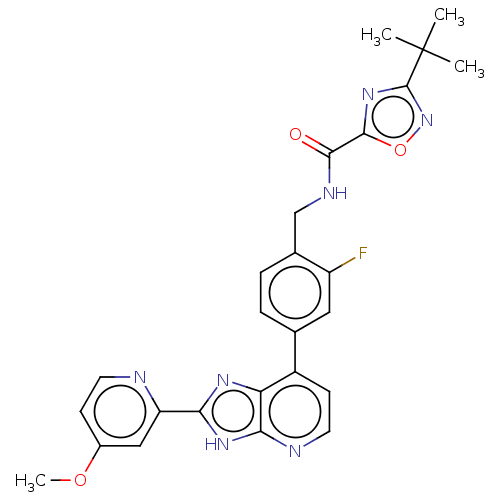
(US11098041, Example 159)Show SMILES COc1ccnc(c1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466206
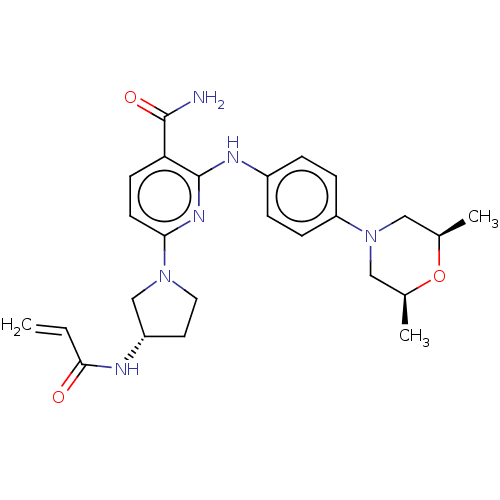
(CHEMBL4281335)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cc1 |r| Show InChI InChI=1S/C25H32N6O3/c1-4-23(32)27-19-11-12-30(15-19)22-10-9-21(24(26)33)25(29-22)28-18-5-7-20(8-6-18)31-13-16(2)34-17(3)14-31/h4-10,16-17,19H,1,11-15H2,2-3H3,(H2,26,33)(H,27,32)(H,28,29)/t16-,17+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466202
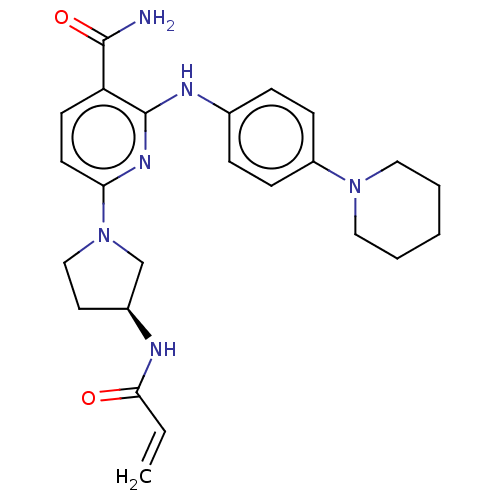
(CHEMBL4278321)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(cc1)N1CCCCC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C24H30N6O2/c1-2-22(31)26-18-12-15-30(16-18)21-11-10-20(23(25)32)24(28-21)27-17-6-8-19(9-7-17)29-13-4-3-5-14-29/h2,6-11,18H,1,3-5,12-16H2,(H2,25,32)(H,26,31)(H,27,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514882
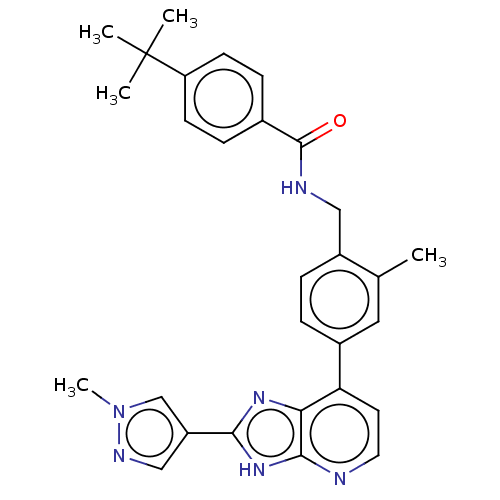
(US11098041, Example 107)Show SMILES Cc1cc(ccc1CNC(=O)c1ccc(cc1)C(C)(C)C)-c1ccnc2[nH]c(nc12)-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514938
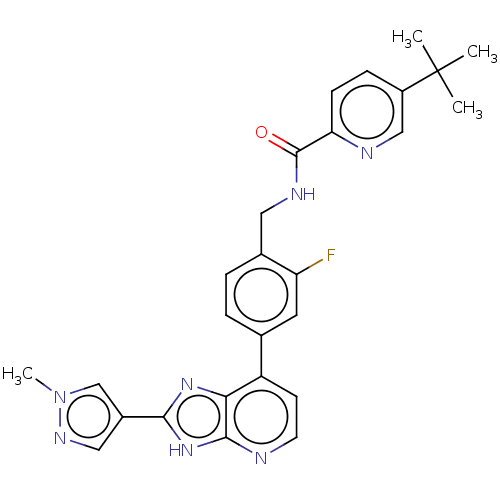
(US11098041, Example 168)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2ccc(cn2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50463727
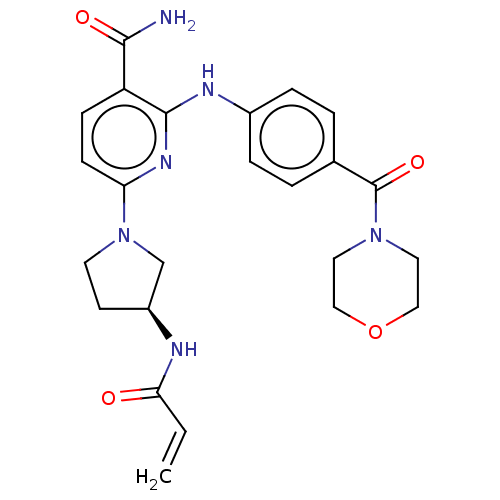
(CHEMBL4245727)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(cc1)C(=O)N1CCOCC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C24H28N6O4/c1-2-21(31)26-18-9-10-30(15-18)20-8-7-19(22(25)32)23(28-20)27-17-5-3-16(4-6-17)24(33)29-11-13-34-14-12-29/h2-8,18H,1,9-15H2,(H2,25,32)(H,26,31)(H,27,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571030
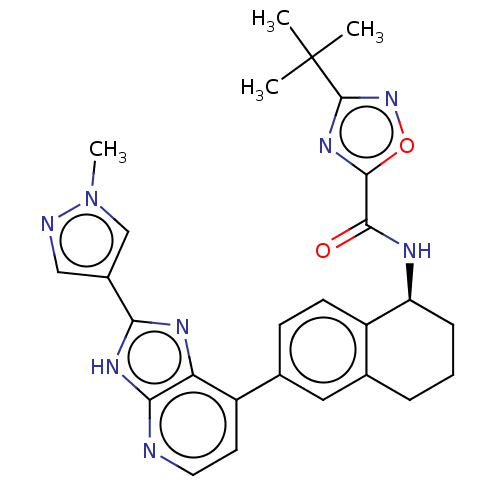
(CHEMBL4869875)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514875
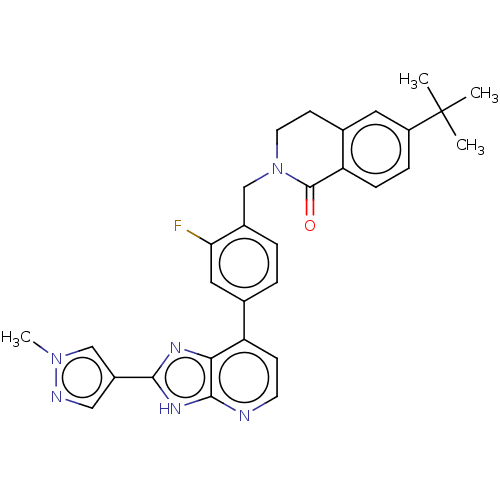
(US11098041, Example 100)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CN2CCc3cc(ccc3C2=O)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466208
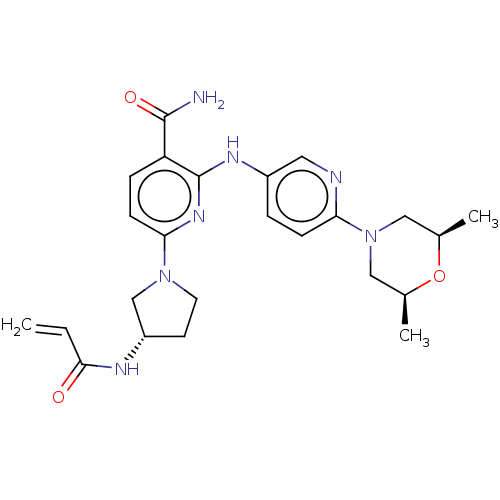
(CHEMBL4282893)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cn1 |r| Show InChI InChI=1S/C24H31N7O3/c1-4-22(32)27-18-9-10-30(14-18)21-8-6-19(23(25)33)24(29-21)28-17-5-7-20(26-11-17)31-12-15(2)34-16(3)13-31/h4-8,11,15-16,18H,1,9-10,12-14H2,2-3H3,(H2,25,33)(H,27,32)(H,28,29)/t15-,16+,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466203
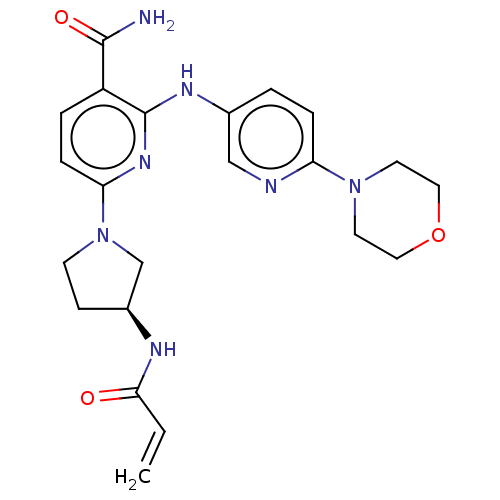
(CHEMBL4286628)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(nc1)N1CCOCC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C22H27N7O3/c1-2-20(30)25-16-7-8-29(14-16)19-6-4-17(21(23)31)22(27-19)26-15-3-5-18(24-13-15)28-9-11-32-12-10-28/h2-6,13,16H,1,7-12,14H2,(H2,23,31)(H,25,30)(H,26,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388189

(CHEMBL2057918)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)nc1 Show InChI InChI=1S/C35H35FN6O3/c1-39-12-14-41(15-13-39)26-8-9-32(37-19-26)38-30-18-25(20-40(2)34(30)44)27-4-3-5-31(28(27)21-43)42-11-10-23-16-24(22-6-7-22)17-29(36)33(23)35(42)45/h3-5,8-11,16-20,22,43H,6-7,12-15,21H2,1-2H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466207
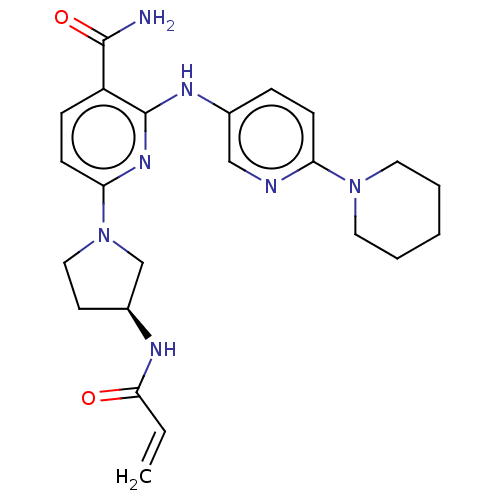
(CHEMBL4278717)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(nc1)N1CCCCC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C23H29N7O2/c1-2-21(31)26-17-10-13-30(15-17)20-9-7-18(22(24)32)23(28-20)27-16-6-8-19(25-14-16)29-11-4-3-5-12-29/h2,6-9,14,17H,1,3-5,10-13,15H2,(H2,24,32)(H,26,31)(H,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514873
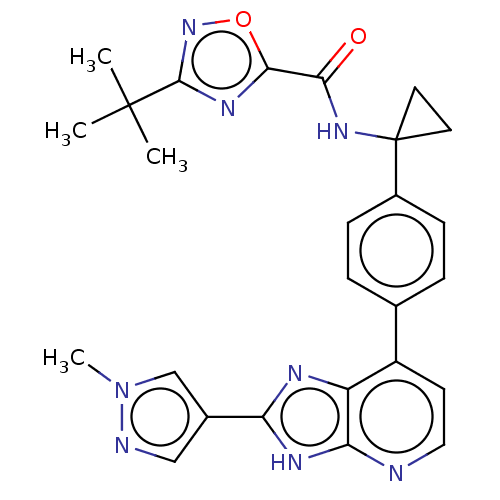
(US11098041, Example 98)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(cc1)C1(CC1)NC(=O)c1nc(no1)C(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466205
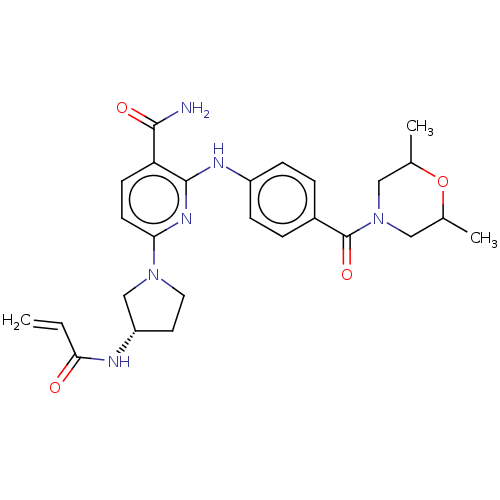
(CHEMBL4285083)Show SMILES CC1CN(CC(C)O1)C(=O)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cc1 |r| Show InChI InChI=1S/C26H32N6O4/c1-4-23(33)28-20-11-12-31(15-20)22-10-9-21(24(27)34)25(30-22)29-19-7-5-18(6-8-19)26(35)32-13-16(2)36-17(3)14-32/h4-10,16-17,20H,1,11-15H2,2-3H3,(H2,27,34)(H,28,33)(H,29,30)/t16?,17?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571039

(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human PBMC |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571039

(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human PBMC |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514783
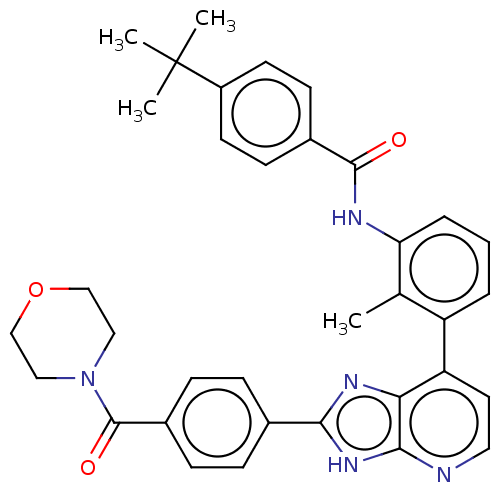
(US11098041, Example 5)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1ccnc2[nH]c(nc12)-c1ccc(cc1)C(=O)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fgr
(Homo sapiens (Human)) | BDBM50571039

(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FGR |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50388189

(CHEMBL2057918)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)nc1 Show InChI InChI=1S/C35H35FN6O3/c1-39-12-14-41(15-13-39)26-8-9-32(37-19-26)38-30-18-25(20-40(2)34(30)44)27-4-3-5-31(28(27)21-43)42-11-10-23-16-24(22-6-7-22)17-29(36)33(23)35(42)45/h3-5,8-11,16-20,22,43H,6-7,12-15,21H2,1-2H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TEC |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514786
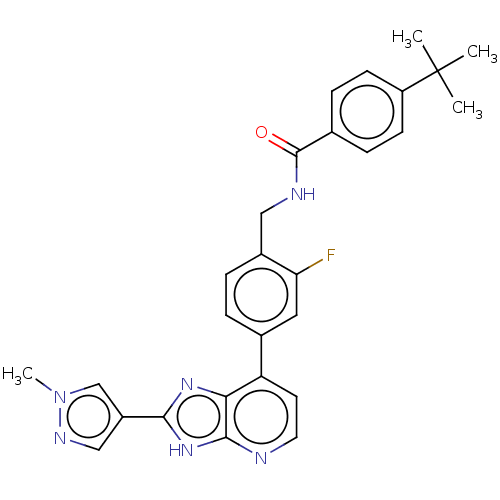
(US11098041, Example 8)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2ccc(cc2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466209
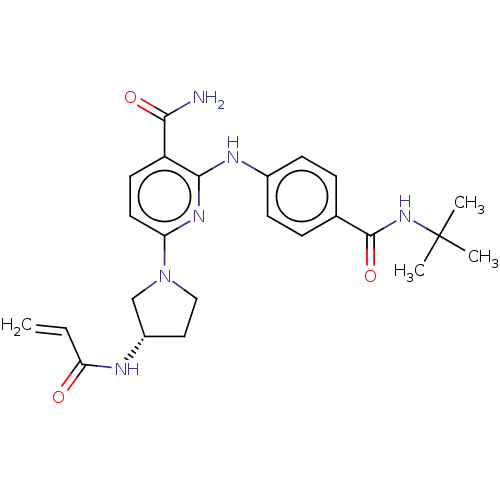
(CHEMBL4290066)Show SMILES CC(C)(C)NC(=O)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cc1 |r| Show InChI InChI=1S/C24H30N6O3/c1-5-20(31)26-17-12-13-30(14-17)19-11-10-18(21(25)32)22(28-19)27-16-8-6-15(7-9-16)23(33)29-24(2,3)4/h5-11,17H,1,12-14H2,2-4H3,(H2,25,32)(H,26,31)(H,27,28)(H,29,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human BTK (8 to 80 residues) using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by off-chip mobility shift assay |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase
(Rattus norvegicus) | BDBM50571039

(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in rat whole blood |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514806
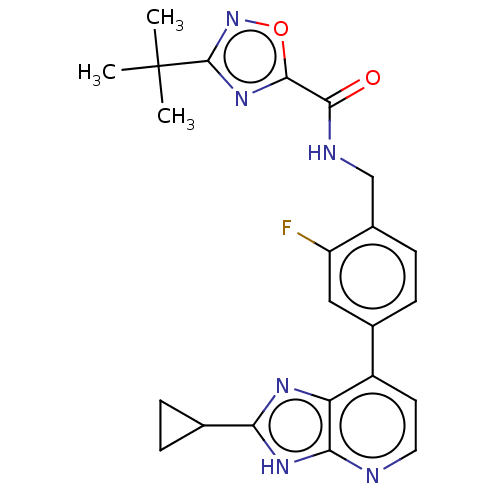
(US11098041, Example 28)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human WBC |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514891

(US11098041, Example 116)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2noc(n2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human PBMC |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466206

(CHEMBL4281335)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cc1 |r| Show InChI InChI=1S/C25H32N6O3/c1-4-23(32)27-19-11-12-30(15-19)22-10-9-21(24(26)33)25(29-22)28-18-5-7-20(8-6-18)31-13-16(2)34-17(3)14-31/h4-10,16-17,19H,1,11-15H2,2-3H3,(H2,26,33)(H,27,32)(H,28,29)/t16-,17+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human PBMC |
Bioorg Med Chem Lett 28: 3307-3311 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.018
BindingDB Entry DOI: 10.7270/Q2J96914 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514793
(US11098041, Example 15)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)C2CCC(CC2)C(C)(C)C)c(F)c1 |(7.1,-4.87,;5.85,-5.78,;4.39,-5.3,;3.48,-6.54,;4.39,-7.79,;5.85,-7.32,;1.94,-6.54,;1.04,-5.3,;-.43,-5.78,;-1.76,-5,;-3.1,-5.78,;-3.1,-7.31,;-1.76,-8.08,;-.43,-7.31,;1.04,-7.79,;-1.76,-3.47,;-3.1,-2.69,;-3.1,-1.15,;-1.76,-.38,;-1.76,1.15,;-.43,1.93,;-.43,3.47,;.91,4.23,;-1.76,4.23,;-1.76,5.78,;-3.1,6.54,;-4.43,5.78,;-4.43,4.23,;-3.1,3.47,;-5.76,6.54,;-5.76,8.08,;-7.1,5.78,;-7.1,7.31,;-.43,-1.15,;.91,-.38,;-.43,-2.69,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514787
(US11098041, Example 9)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1ccnc2[nH]c(nc12)-c1cnn(C)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514897

(US11098041, Example 124)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)-c1cnn(c1)C1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human PBMC |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data