

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122995 ((S)-3-(2-(1-methoxypropan-2-ylamino)pyridin-4-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122996 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyrimidin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215268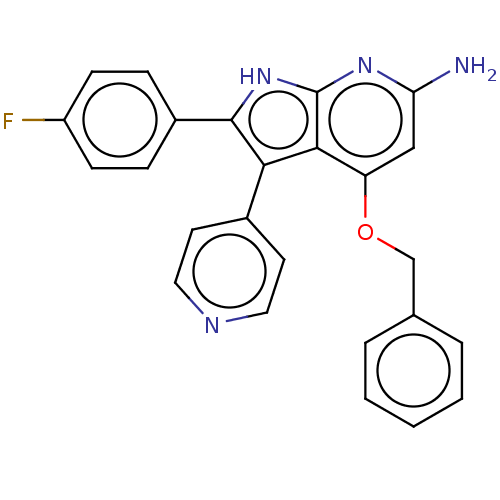 (CHEMBL325211) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50122997 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyridin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50067495 (2-(4-Fluoro-phenyl)-4-(3-methoxy-benzyloxy)-3-pyri...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50122995 ((S)-3-(2-(1-methoxypropan-2-ylamino)pyridin-4-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122997 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyridin-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215161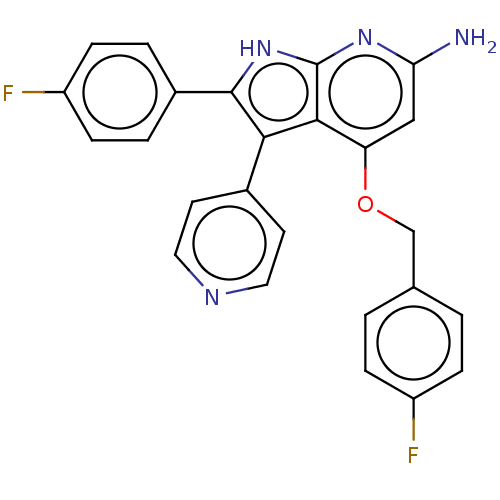 (CHEMBL324468) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50123004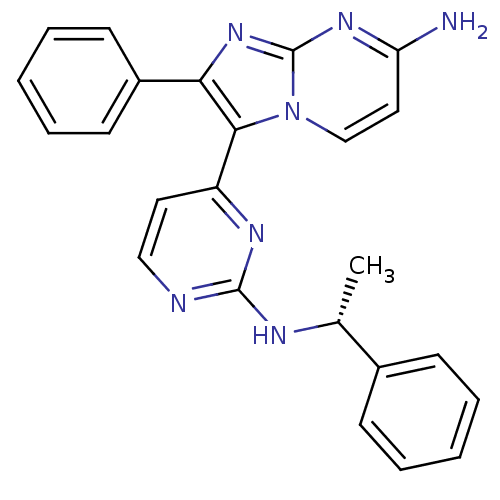 (2-Phenyl-3-[2-((R)-1-phenyl-ethylamino)-pyrimidin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50123000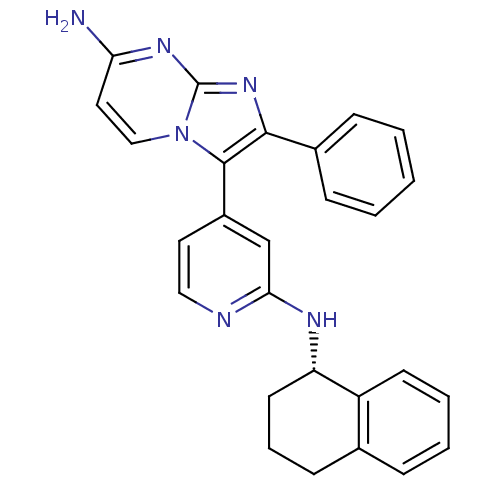 (2-Phenyl-3-{2-[(S)-(1,2,3,4-tetrahydro-naphthalen-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215299 (CHEMBL263536) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123003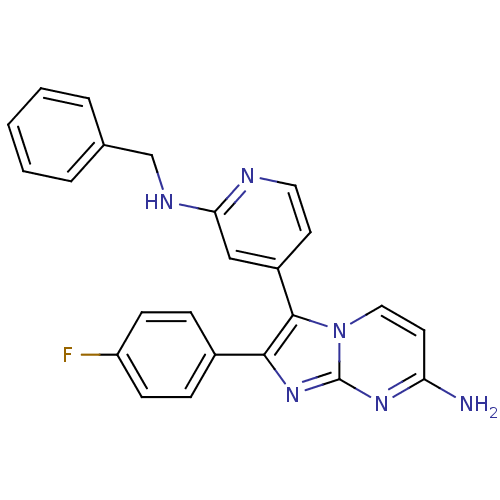 (3-(2-(benzylamino)pyridin-4-yl)-2-(4-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215274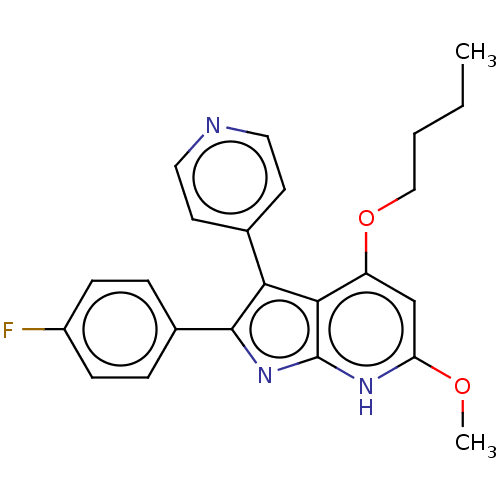 (CHEMBL332917) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50123003 (3-(2-(benzylamino)pyridin-4-yl)-2-(4-fluorophenyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545768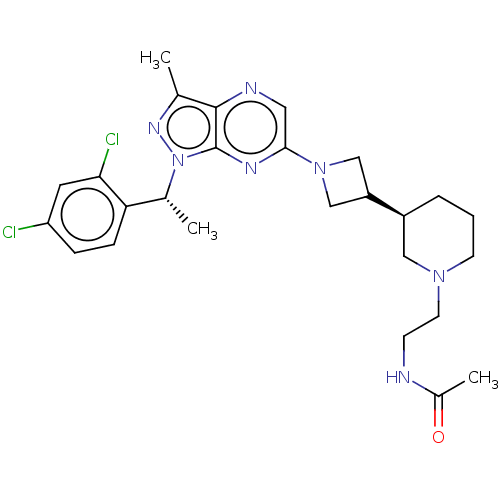 (CHEMBL4641127) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215267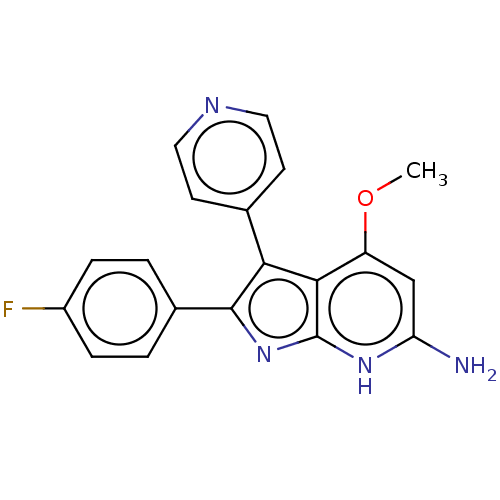 (CHEMBL115769 | RWJ-68354) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM15457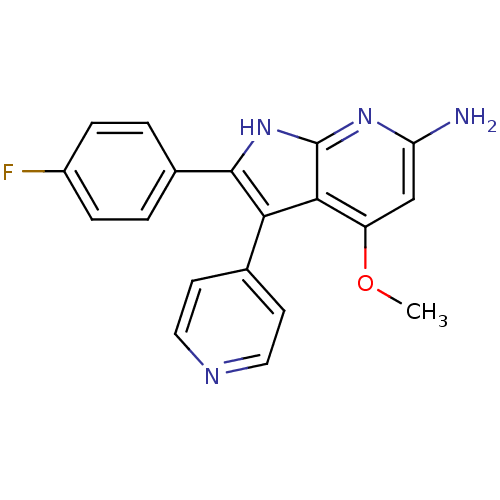 (2-(4-fluorophenyl)-4-methoxy-3-(pyridin-4-yl)-1H-p...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545779 (CHEMBL4642563) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50122996 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123000 (2-Phenyl-3-{2-[(S)-(1,2,3,4-tetrahydro-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215272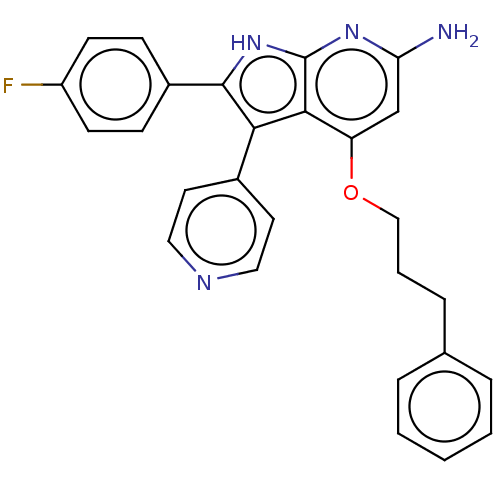 (CHEMBL115778) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215158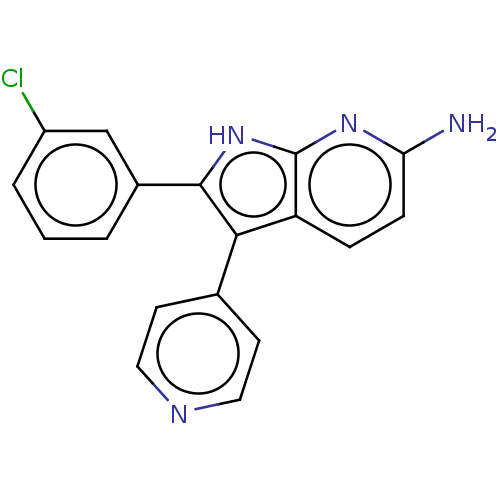 (CHEMBL333823) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545782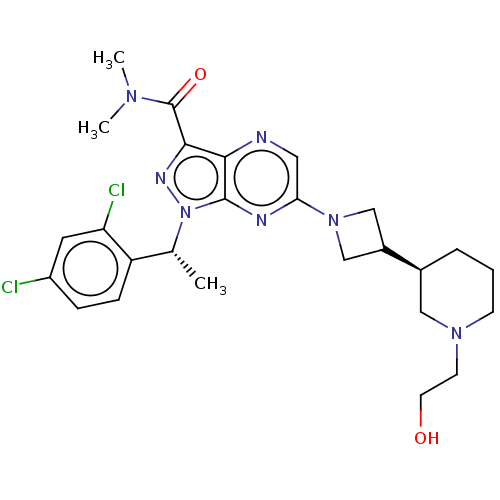 (CHEMBL4637695) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215154 (CHEMBL112691) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545769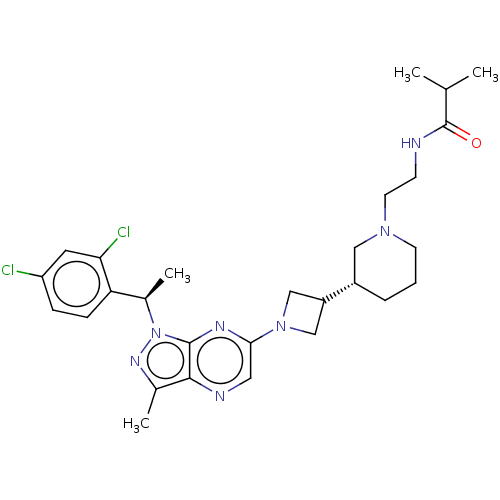 (CHEMBL4645325) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545771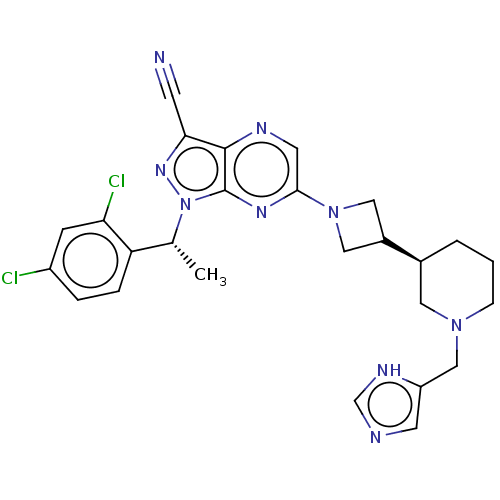 (CHEMBL4639600) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545778 (CHEMBL4637143) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123002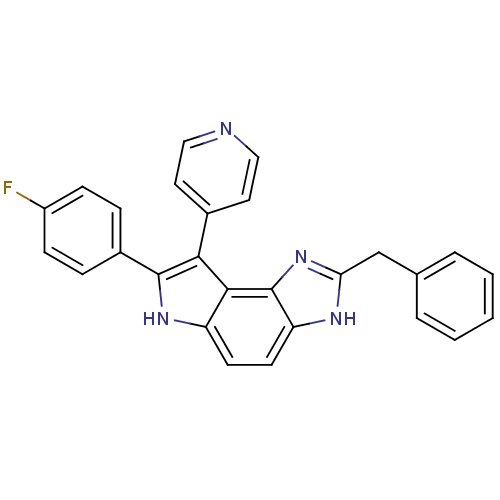 (2-Benzyl-7-(4-fluoro-phenyl)-8-pyridin-4-yl-3,6-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545762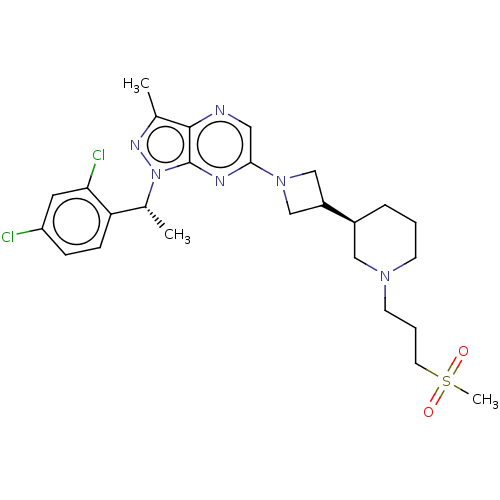 (CHEMBL4647117) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374633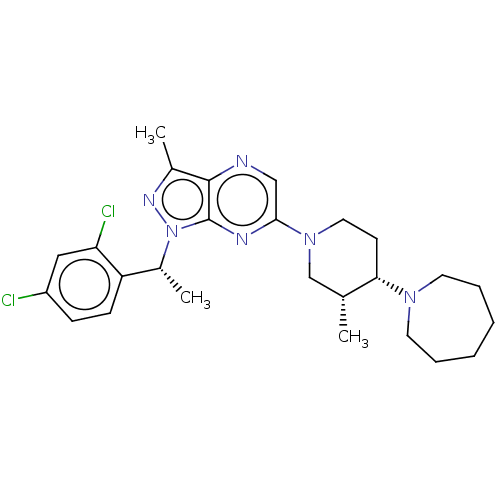 (6-((3R,4S)-4-(Azepan-1-yl)-3-methylpiperidin-1-yl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545770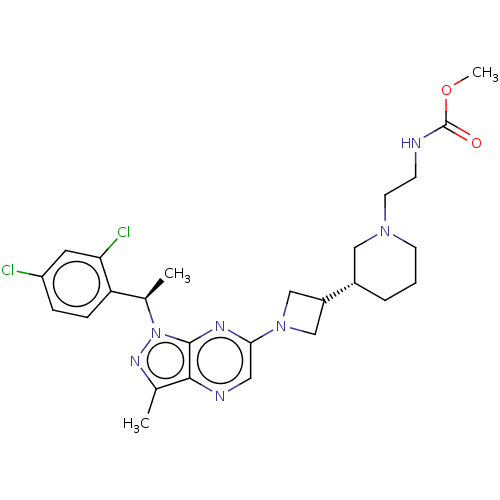 (CHEMBL4641595) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545777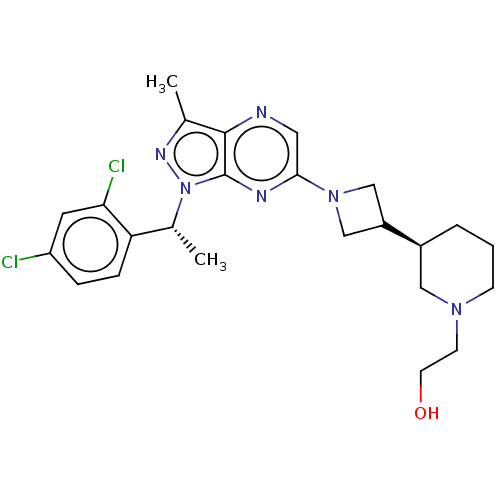 (CHEMBL4634054) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50045333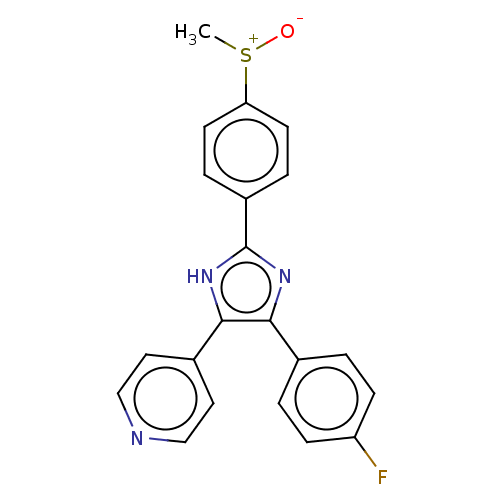 (CHEBI:90705 | SB-203580) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545781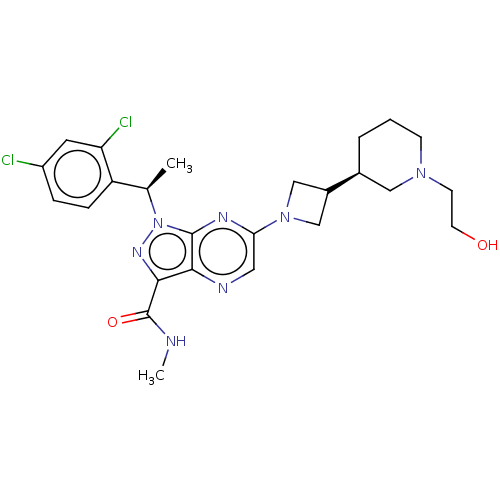 (CHEMBL4633133) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374652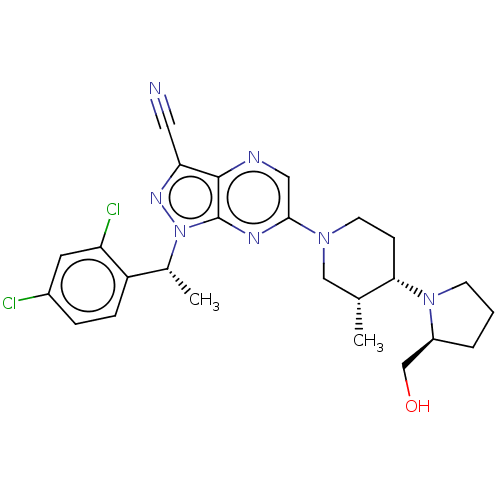 (1-((R)-1-(2,4-Dichlorophenyl)ethyl)-6-((3R,4S)-4-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545773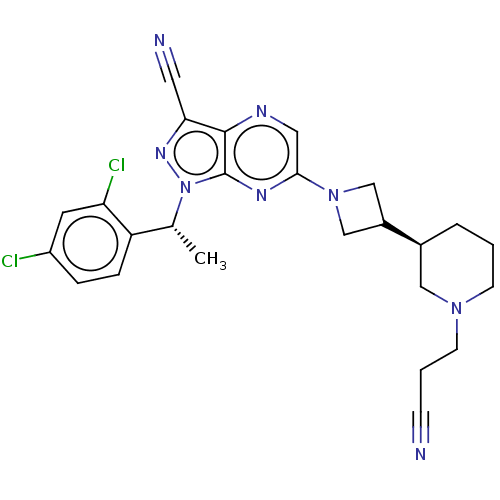 (CHEMBL4633494) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122998 (3-(2-Methylsulfanyl-pyrimidin-4-yl)-2-phenyl-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545759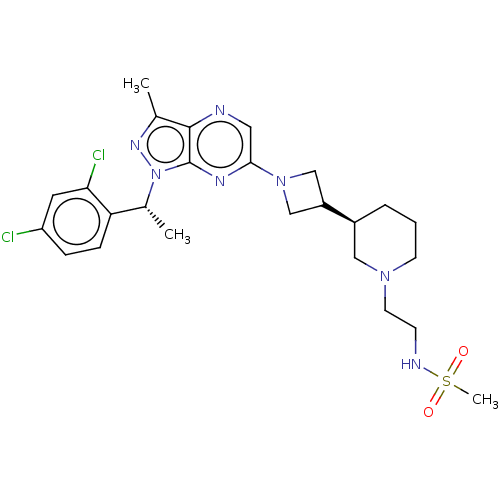 (CHEMBL4647188) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215159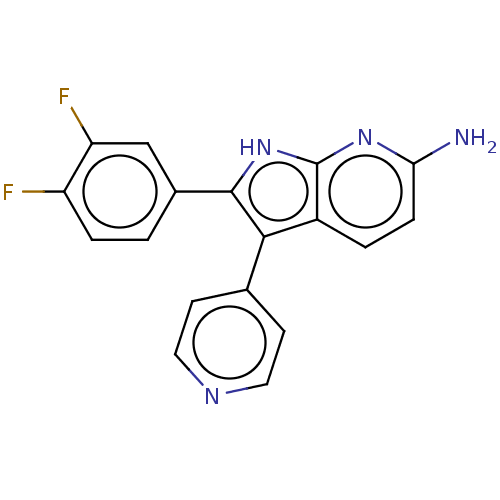 (CHEMBL112274) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545760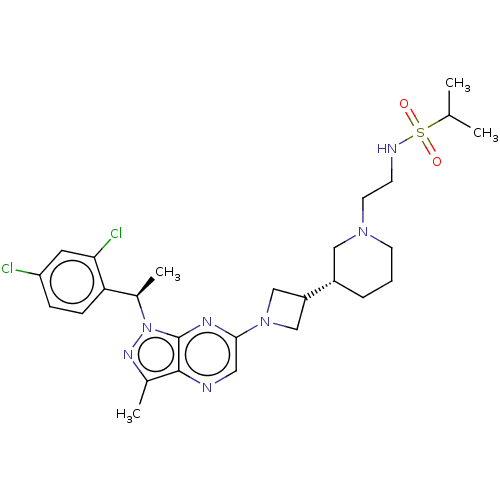 (CHEMBL4636749) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516634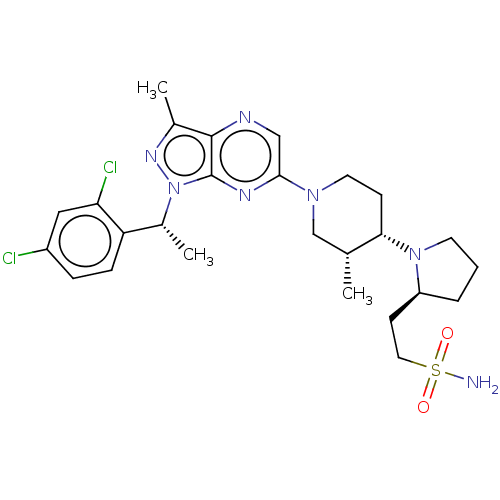 (CHEMBL4473604) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374650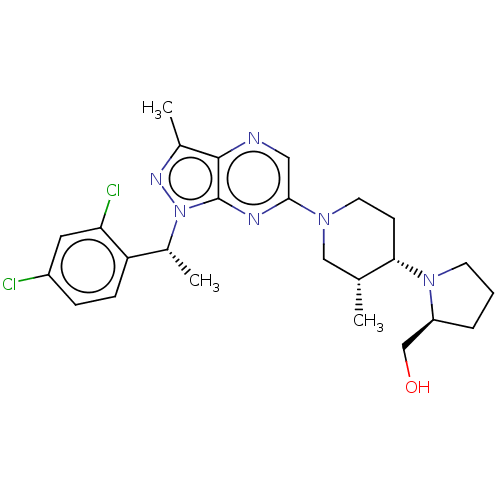 (((S)-1-((3R,4S)-1-(1-((R)-1-(2,4-Dichlorophenyl)et...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545778 (CHEMBL4637143) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human Treg cells assessed as inhibition of CCL22-mediated chemotaxis preincubated for 30 mins followed by CCL22 additi... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516626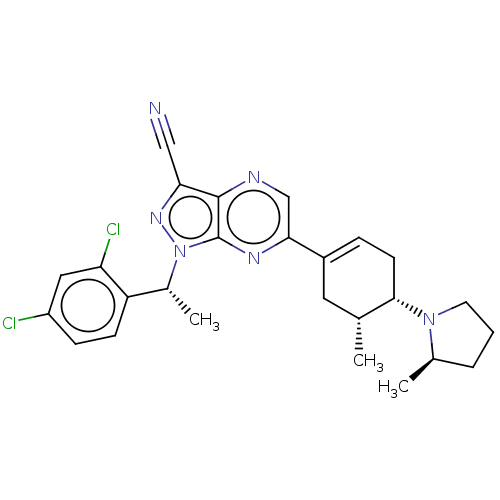 (CHEMBL4475665) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545766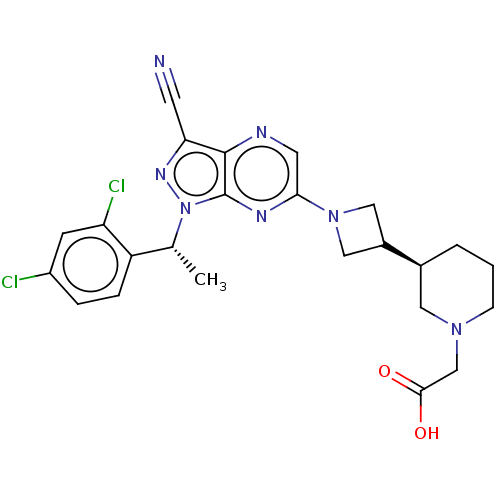 (CHEMBL4645037) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516632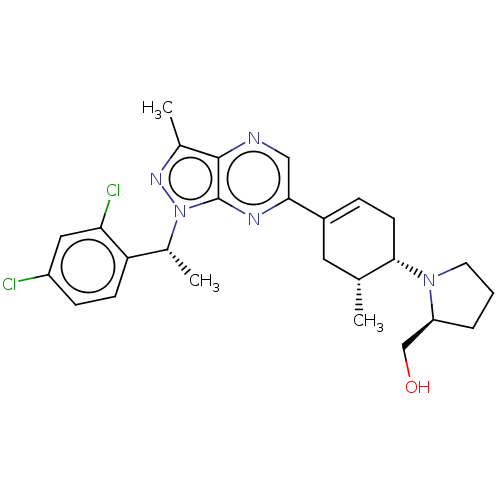 (CHEMBL4443688) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545779 (CHEMBL4642563) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in human CCRF-CEM cells assessed as inhibition of CCL22-mediated chemotaxis preincubated for 30 mins followed by CCL22 ad... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50516623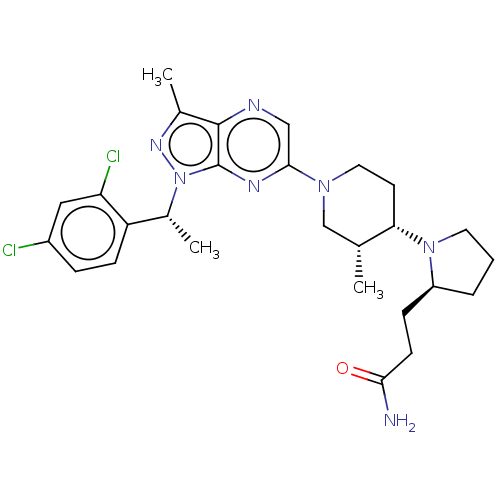 (CHEMBL4575089) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50215262 (CHEMBL112273) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of TNF-alpha production by lipopolysaccharide-stimulated human peripheral blood mononuclear cells | Bioorg Med Chem Lett 8: 3335-40 (1998) BindingDB Entry DOI: 10.7270/Q25T3NNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123004 (2-Phenyl-3-[2-((R)-1-phenyl-ethylamino)-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |