 Found 114 hits with Last Name = 'adile' and Initial = 'aa'
Found 114 hits with Last Name = 'adile' and Initial = 'aa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603512
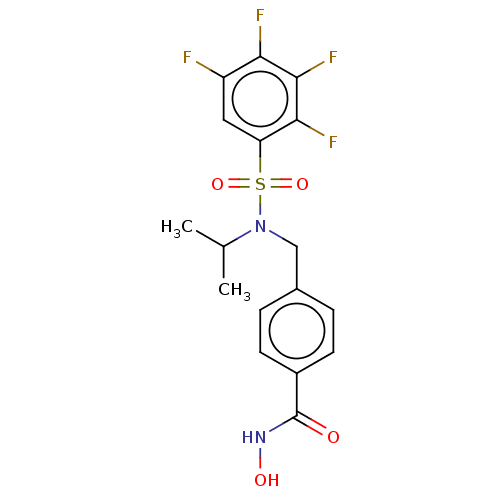
(CHEMBL5177475)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595960

(CHEMBL5192865)Show SMILES ONC(=O)c1ccc(CN(C2CC2)S(=O)(=O)c2cc(F)c(F)c(F)c2F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM110036
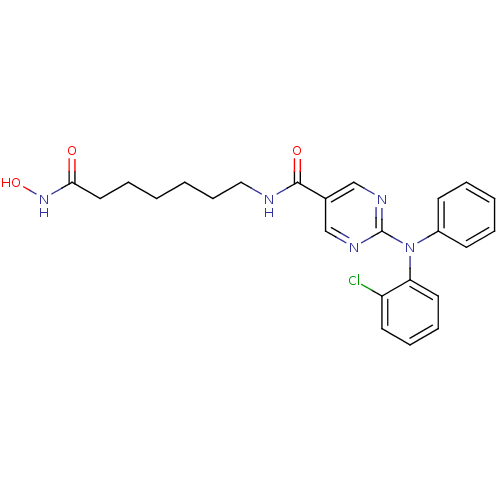
(US8609678, 2-((2-chlorophenyl)(phenyl)amino)-N-(7-...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C24H26ClN5O3/c25-20-12-7-8-13-21(20)30(19-10-4-3-5-11-19)24-27-16-18(17-28-24)23(32)26-15-9-2-1-6-14-22(31)29-33/h3-5,7-8,10-13,16-17,33H,1-2,6,9,14-15H2,(H,26,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
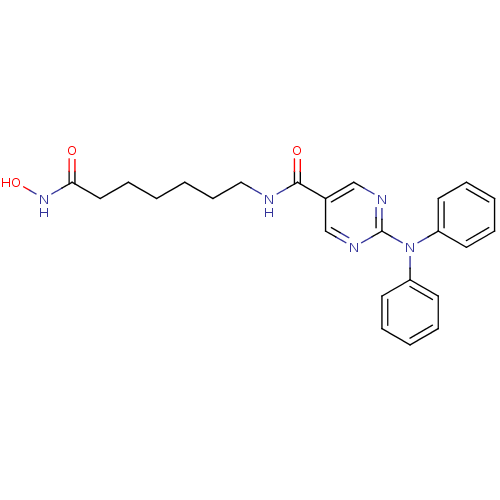
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595950
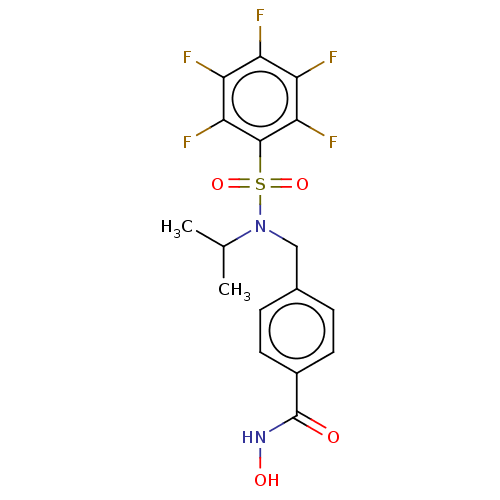
(CHEMBL5179295)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1c(F)c(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603512

(CHEMBL5177475)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595960

(CHEMBL5192865)Show SMILES ONC(=O)c1ccc(CN(C2CC2)S(=O)(=O)c2cc(F)c(F)c(F)c2F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50398716
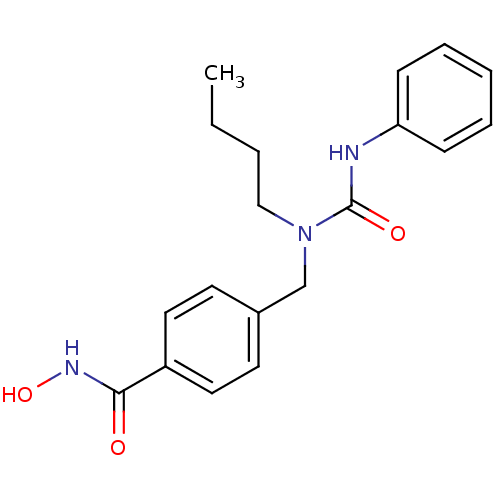
(CHEMBL2179618 | US10227295, Compound 5g | US940985...)Show InChI InChI=1S/C19H23N3O3/c1-2-3-13-22(19(24)20-17-7-5-4-6-8-17)14-15-9-11-16(12-10-15)18(23)21-25/h4-12,25H,2-3,13-14H2,1H3,(H,20,24)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM110036

(US8609678, 2-((2-chlorophenyl)(phenyl)amino)-N-(7-...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C24H26ClN5O3/c25-20-12-7-8-13-21(20)30(19-10-4-3-5-11-19)24-27-16-18(17-28-24)23(32)26-15-9-2-1-6-14-22(31)29-33/h3-5,7-8,10-13,16-17,33H,1-2,6,9,14-15H2,(H,26,32)(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50555683
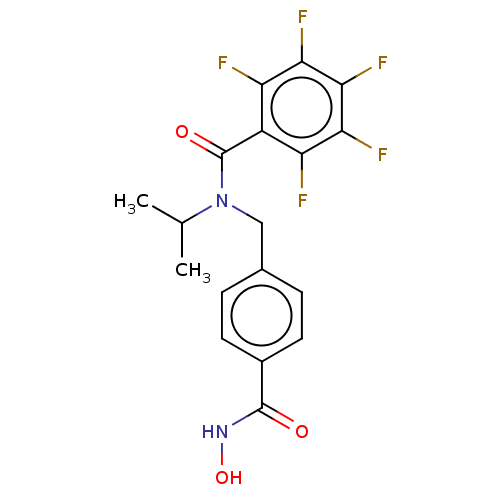
(CHEMBL4763401)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)C(=O)c1c(F)c(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603511
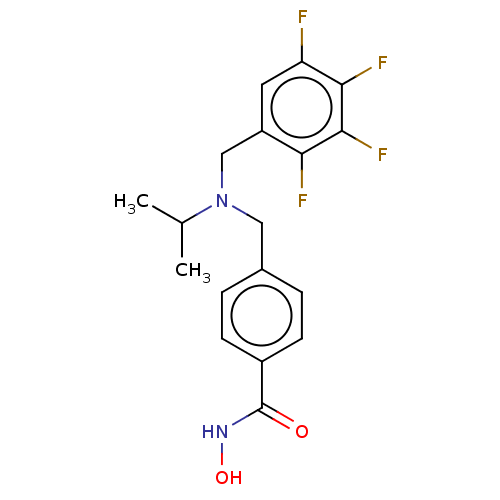
(CHEMBL5173782)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)Cc1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603516
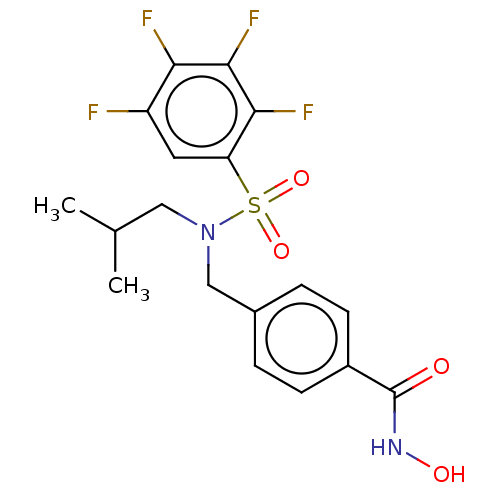
(CHEMBL5202595)Show SMILES CC(C)CN(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603517
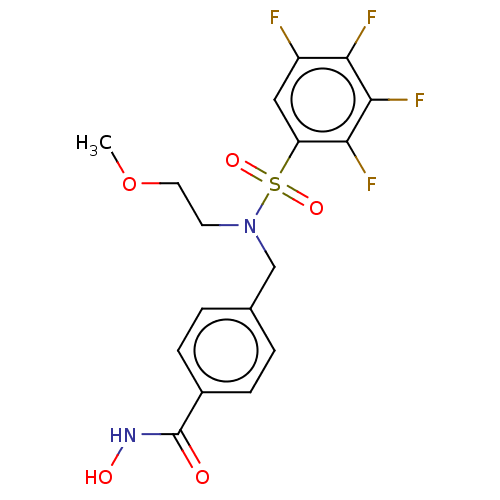
(CHEMBL5191960)Show SMILES COCCN(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603519
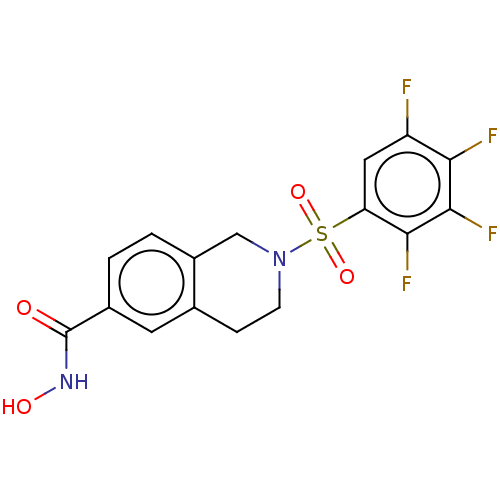
(CHEMBL5202970)Show SMILES ONC(=O)c1ccc2CN(CCc2c1)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603514
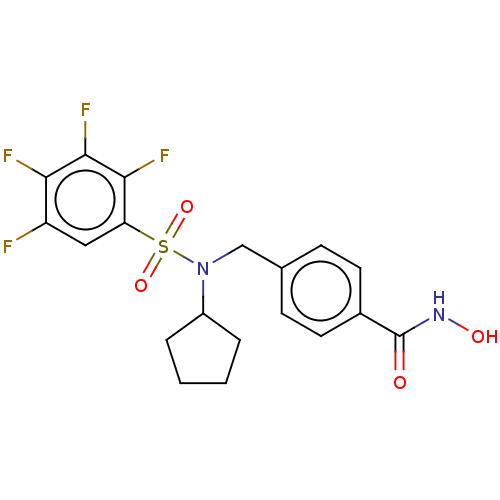
(CHEMBL5199669)Show SMILES ONC(=O)c1ccc(CN(C2CCCC2)S(=O)(=O)c2cc(F)c(F)c(F)c2F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50603513
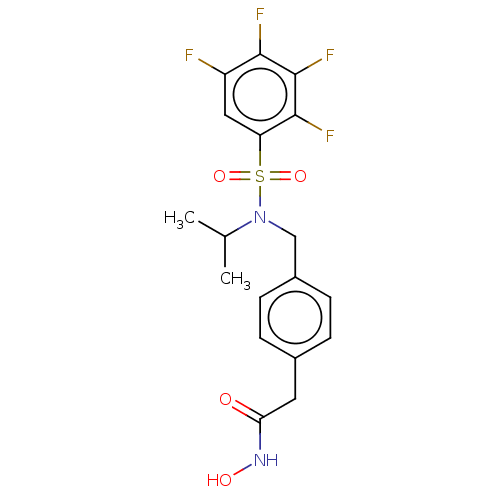
(CHEMBL5184540)Show SMILES CC(C)N(Cc1ccc(CC(=O)NO)cc1)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526254
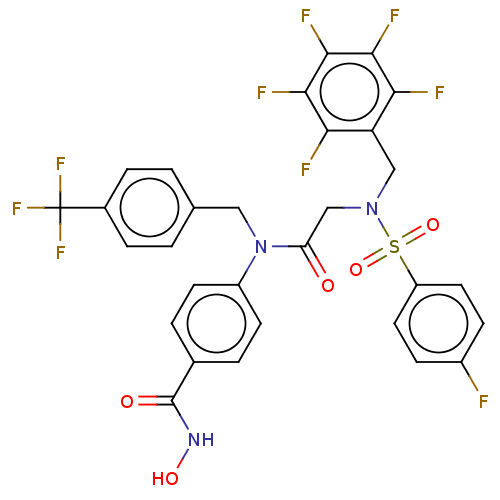
(CHEMBL4563091)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1c(F)c(F)c(F)c(F)c1F)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C30H20F9N3O5S/c31-19-7-11-21(12-8-19)48(46,47)41(14-22-24(32)26(34)28(36)27(35)25(22)33)15-23(43)42(20-9-3-17(4-10-20)29(44)40-45)13-16-1-5-18(6-2-16)30(37,38)39/h1-12,45H,13-15H2,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526249
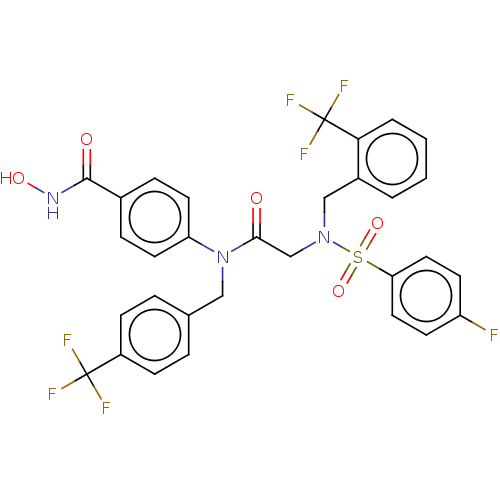
(CHEMBL4561439)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C31H24F7N3O5S/c32-24-11-15-26(16-12-24)47(45,46)40(18-22-3-1-2-4-27(22)31(36,37)38)19-28(42)41(25-13-7-21(8-14-25)29(43)39-44)17-20-5-9-23(10-6-20)30(33,34)35/h1-16,44H,17-19H2,(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603515
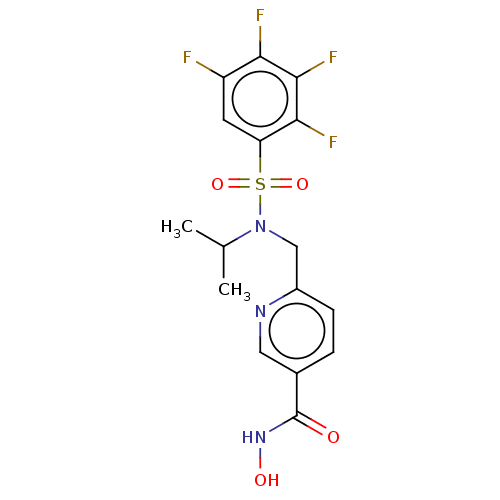
(CHEMBL5195141)Show SMILES CC(C)N(Cc1ccc(cn1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50603513

(CHEMBL5184540)Show SMILES CC(C)N(Cc1ccc(CC(=O)NO)cc1)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526255
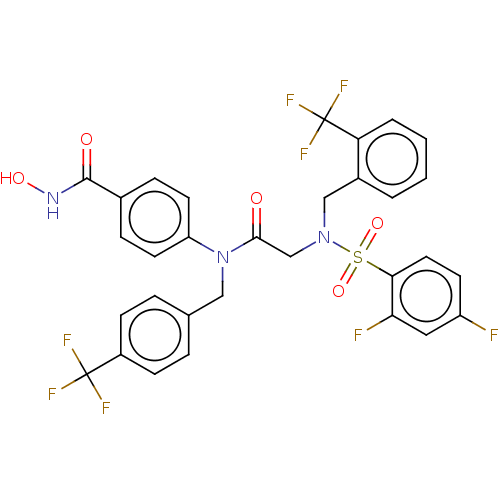
(CHEMBL4447317)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C31H23F8N3O5S/c32-23-11-14-27(26(33)15-23)48(46,47)41(17-21-3-1-2-4-25(21)31(37,38)39)18-28(43)42(24-12-7-20(8-13-24)29(44)40-45)16-19-5-9-22(10-6-19)30(34,35)36/h1-15,45H,16-18H2,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM110036

(US8609678, 2-((2-chlorophenyl)(phenyl)amino)-N-(7-...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C24H26ClN5O3/c25-20-12-7-8-13-21(20)30(19-10-4-3-5-11-19)24-27-16-18(17-28-24)23(32)26-15-9-2-1-6-14-22(31)29-33/h3-5,7-8,10-13,16-17,33H,1-2,6,9,14-15H2,(H,26,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526250
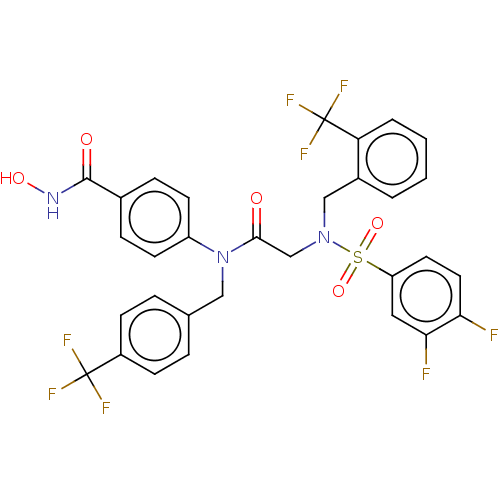
(CHEMBL4578694)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C31H23F8N3O5S/c32-26-14-13-24(15-27(26)33)48(46,47)41(17-21-3-1-2-4-25(21)31(37,38)39)18-28(43)42(23-11-7-20(8-12-23)29(44)40-45)16-19-5-9-22(10-6-19)30(34,35)36/h1-15,45H,16-18H2,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50519452
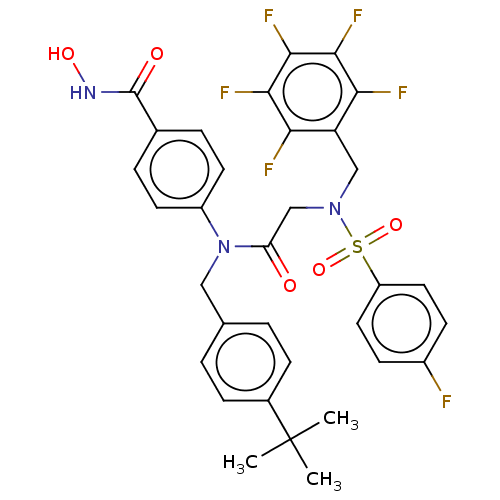
(CHEMBL4520400)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2c(F)c(F)c(F)c(F)c2F)S(=O)(=O)c2ccc(F)cc2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C33H29F6N3O5S/c1-33(2,3)21-8-4-19(5-9-21)16-42(23-12-6-20(7-13-23)32(44)40-45)26(43)18-41(48(46,47)24-14-10-22(34)11-15-24)17-25-27(35)29(37)31(39)30(38)28(25)36/h4-15,45H,16-18H2,1-3H3,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526254

(CHEMBL4563091)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1c(F)c(F)c(F)c(F)c1F)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C30H20F9N3O5S/c31-19-7-11-21(12-8-19)48(46,47)41(14-22-24(32)26(34)28(36)27(35)25(22)33)15-23(43)42(20-9-3-17(4-10-20)29(44)40-45)13-16-1-5-18(6-2-16)30(37,38)39/h1-12,45H,13-15H2,(H,40,44) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526251
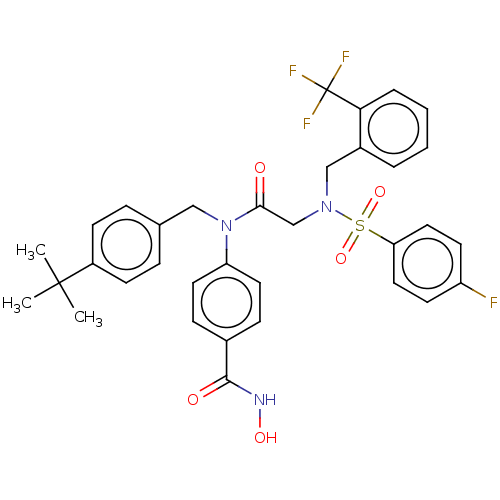
(CHEMBL4546897)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2ccccc2C(F)(F)F)S(=O)(=O)c2ccc(F)cc2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C34H33F4N3O5S/c1-33(2,3)26-12-8-23(9-13-26)20-41(28-16-10-24(11-17-28)32(43)39-44)31(42)22-40(47(45,46)29-18-14-27(35)15-19-29)21-25-6-4-5-7-30(25)34(36,37)38/h4-19,44H,20-22H2,1-3H3,(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50398716

(CHEMBL2179618 | US10227295, Compound 5g | US940985...)Show InChI InChI=1S/C19H23N3O3/c1-2-3-13-22(19(24)20-17-7-5-4-6-8-17)14-15-9-11-16(12-10-15)18(23)21-25/h4-12,25H,2-3,13-14H2,1H3,(H,20,24)(H,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526251

(CHEMBL4546897)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2ccccc2C(F)(F)F)S(=O)(=O)c2ccc(F)cc2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C34H33F4N3O5S/c1-33(2,3)26-12-8-23(9-13-26)20-41(28-16-10-24(11-17-28)32(43)39-44)31(42)22-40(47(45,46)29-18-14-27(35)15-19-29)21-25-6-4-5-7-30(25)34(36,37)38/h4-19,44H,20-22H2,1-3H3,(H,39,43) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526252
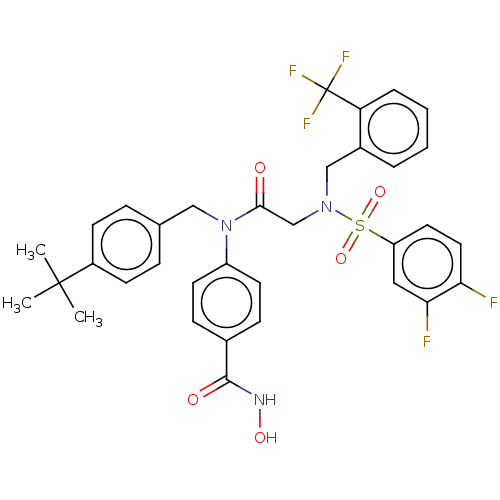
(CHEMBL4534860)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2ccccc2C(F)(F)F)S(=O)(=O)c2ccc(F)c(F)c2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C34H32F5N3O5S/c1-33(2,3)25-12-8-22(9-13-25)19-42(26-14-10-23(11-15-26)32(44)40-45)31(43)21-41(20-24-6-4-5-7-28(24)34(37,38)39)48(46,47)27-16-17-29(35)30(36)18-27/h4-18,45H,19-21H2,1-3H3,(H,40,44) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526253
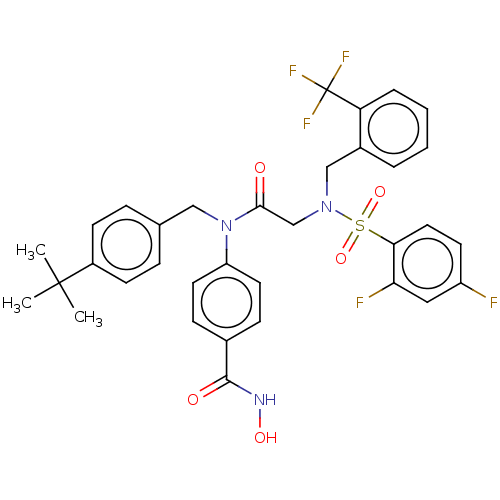
(CHEMBL4580317)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2ccccc2C(F)(F)F)S(=O)(=O)c2ccc(F)cc2F)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C34H32F5N3O5S/c1-33(2,3)25-12-8-22(9-13-25)19-42(27-15-10-23(11-16-27)32(44)40-45)31(43)21-41(20-24-6-4-5-7-28(24)34(37,38)39)48(46,47)30-17-14-26(35)18-29(30)36/h4-18,45H,19-21H2,1-3H3,(H,40,44) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526253

(CHEMBL4580317)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2ccccc2C(F)(F)F)S(=O)(=O)c2ccc(F)cc2F)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C34H32F5N3O5S/c1-33(2,3)25-12-8-22(9-13-25)19-42(27-15-10-23(11-16-27)32(44)40-45)31(43)21-41(20-24-6-4-5-7-28(24)34(37,38)39)48(46,47)30-17-14-26(35)18-29(30)36/h4-18,45H,19-21H2,1-3H3,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526249

(CHEMBL4561439)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C31H24F7N3O5S/c32-24-11-15-26(16-12-24)47(45,46)40(18-22-3-1-2-4-27(22)31(36,37)38)19-28(42)41(25-13-7-21(8-14-25)29(43)39-44)17-20-5-9-23(10-6-20)30(33,34)35/h1-16,44H,17-19H2,(H,39,43) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50603512

(CHEMBL5177475)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1cc(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526255

(CHEMBL4447317)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C31H23F8N3O5S/c32-23-11-14-27(26(33)15-23)48(46,47)41(17-21-3-1-2-4-25(21)31(37,38)39)18-28(43)42(24-12-7-20(8-13-24)29(44)40-45)16-19-5-9-22(10-6-19)30(34,35)36/h1-15,45H,16-18H2,(H,40,44) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526252

(CHEMBL4534860)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2ccccc2C(F)(F)F)S(=O)(=O)c2ccc(F)c(F)c2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C34H32F5N3O5S/c1-33(2,3)25-12-8-22(9-13-25)19-42(26-14-10-23(11-15-26)32(44)40-45)31(43)21-41(20-24-6-4-5-7-28(24)34(37,38)39)48(46,47)27-16-17-29(35)30(36)18-27/h4-18,45H,19-21H2,1-3H3,(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50526250

(CHEMBL4578694)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)c(F)c1 Show InChI InChI=1S/C31H23F8N3O5S/c32-26-14-13-24(15-27(26)33)48(46,47)41(17-21-3-1-2-4-25(21)31(37,38)39)18-28(43)42(23-11-7-20(8-12-23)29(44)40-45)16-19-5-9-22(10-6-19)30(34,35)36/h1-15,45H,16-18H2,(H,40,44) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 396 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50603518
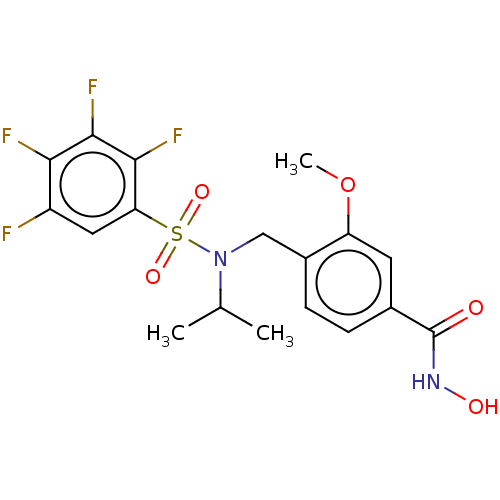
(CHEMBL5208971)Show SMILES COc1cc(ccc1CN(C(C)C)S(=O)(=O)c1cc(F)c(F)c(F)c1F)C(=O)NO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 474 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 497 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50519452

(CHEMBL4520400)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2c(F)c(F)c(F)c(F)c2F)S(=O)(=O)c2ccc(F)cc2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C33H29F6N3O5S/c1-33(2,3)21-8-4-19(5-9-21)16-42(23-12-6-20(7-13-23)32(44)40-45)26(43)18-41(48(46,47)24-14-10-22(34)11-15-24)17-25-27(35)29(37)31(39)30(38)28(25)36/h4-15,45H,16-18H2,1-3H3,(H,40,44) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 636 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC11 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50595950

(CHEMBL5179295)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1c(F)c(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 653 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50519452

(CHEMBL4520400)Show SMILES CC(C)(C)c1ccc(CN(C(=O)CN(Cc2c(F)c(F)c(F)c(F)c2F)S(=O)(=O)c2ccc(F)cc2)c2ccc(cc2)C(=O)NO)cc1 Show InChI InChI=1S/C33H29F6N3O5S/c1-33(2,3)21-8-4-19(5-9-21)16-42(23-12-6-20(7-13-23)32(44)40-45)26(43)18-41(48(46,47)24-14-10-22(34)11-15-24)17-25-27(35)29(37)31(39)30(38)28(25)36/h4-15,45H,16-18H2,1-3H3,(H,40,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 654 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50555683

(CHEMBL4763401)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)C(=O)c1c(F)c(F)c(F)c(F)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 876 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50603511

(CHEMBL5173782)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)Cc1cc(F)c(F)c(F)c1F | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 899 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50595950

(CHEMBL5179295)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1c(F)c(F)c(F)c(F)c1F | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 932 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50595950

(CHEMBL5179295)Show SMILES CC(C)N(Cc1ccc(cc1)C(=O)NO)S(=O)(=O)c1c(F)c(F)c(F)c(F)c1F | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 941 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01585
BindingDB Entry DOI: 10.7270/Q2QV3RKM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50526249

(CHEMBL4561439)Show SMILES ONC(=O)c1ccc(cc1)N(Cc1ccc(cc1)C(F)(F)F)C(=O)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C31H24F7N3O5S/c32-24-11-15-26(16-12-24)47(45,46)40(18-22-3-1-2-4-27(22)31(36,37)38)19-28(42)41(25-13-7-21(8-14-25)29(43)39-44)17-20-5-9-23(10-6-20)30(33,34)35/h1-16,44H,17-19H2,(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC8 expressed in SF9 baculoviral system using FAM-labeled acetylated peptide as substrate by EMSA |
J Med Chem 62: 2651-2665 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01957
BindingDB Entry DOI: 10.7270/Q2SQ93VV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data