

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50463665 (CHEMBL4240365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463663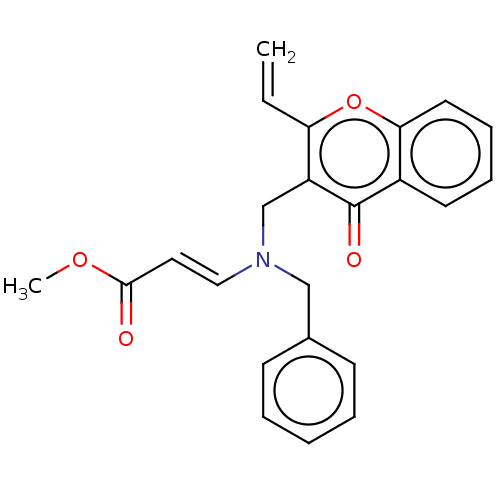 (CHEMBL4244573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463658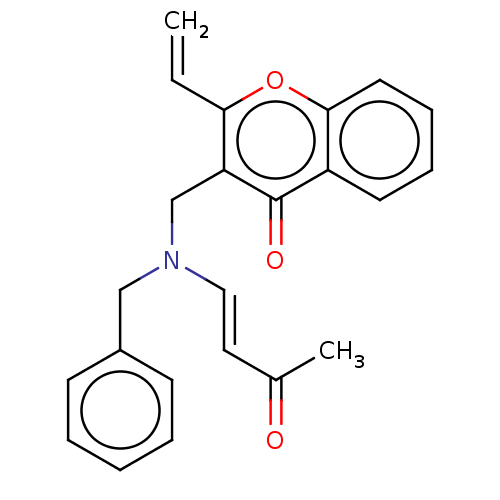 (CHEMBL4246032) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Linewe... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50130915 (CHEBI:3122 | CHEMBL1231178) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463665 (CHEMBL4240365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463658 (CHEMBL4246032) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463663 (CHEMBL4244573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463656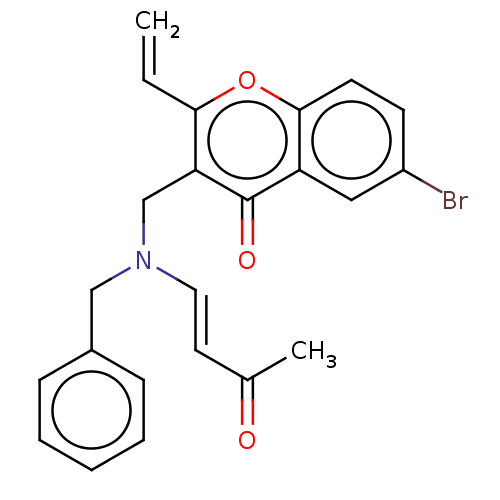 (CHEMBL4250287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463664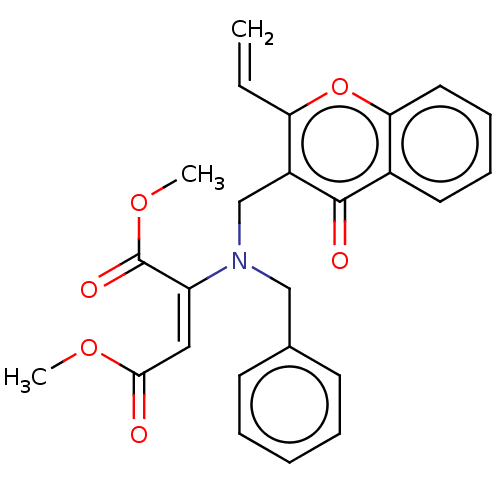 (CHEMBL4250143) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463661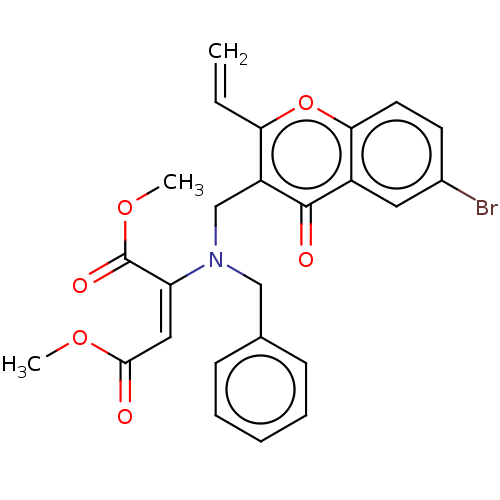 (CHEMBL4238512) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463664 (CHEMBL4250143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463661 (CHEMBL4238512) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463658 (CHEMBL4246032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463663 (CHEMBL4244573) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463656 (CHEMBL4250287) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463666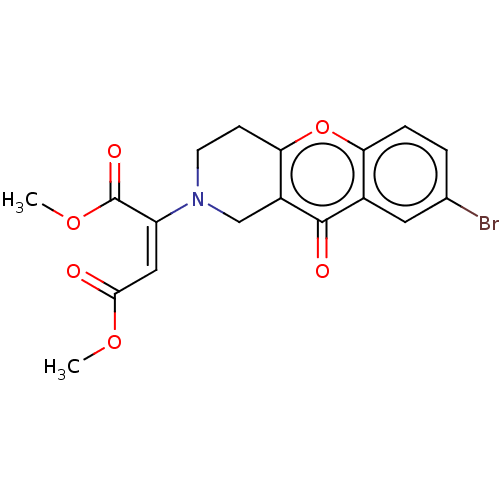 (CHEMBL4238937) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463667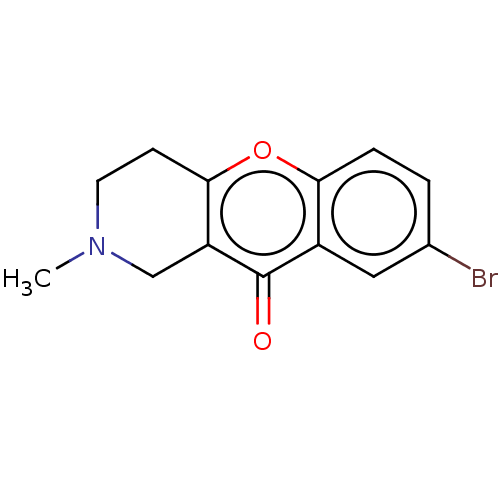 (CHEMBL4248378) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463668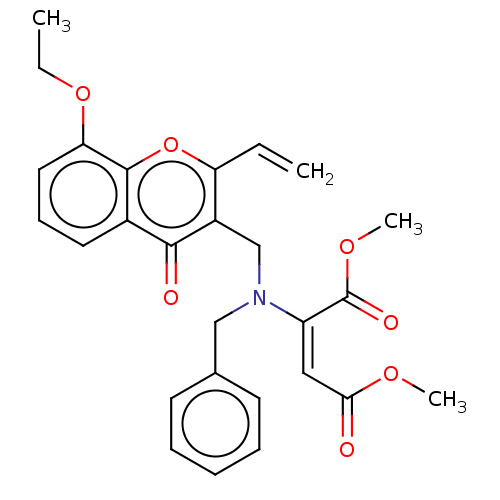 (CHEMBL4242675) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463669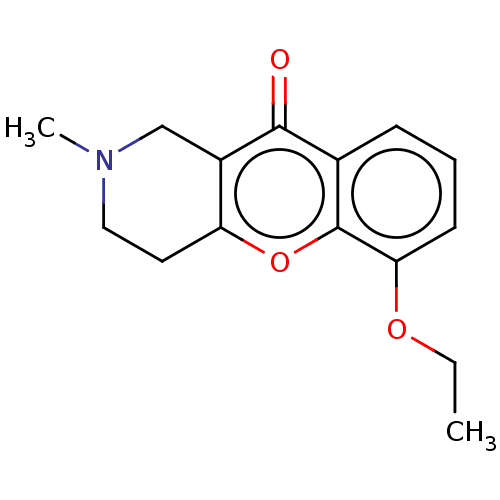 (CHEMBL4250510) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463660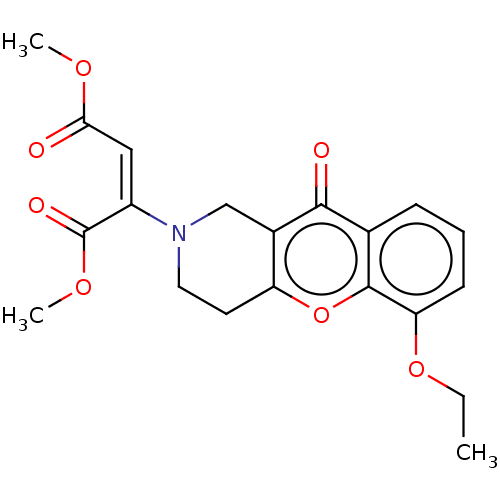 (CHEMBL4250314) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463659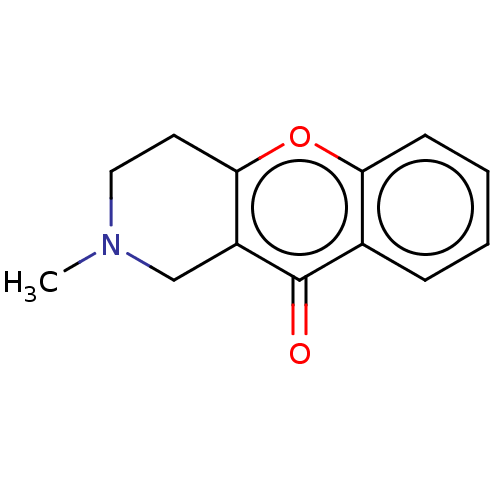 (CHEMBL4247106) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463666 (CHEMBL4238937) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463670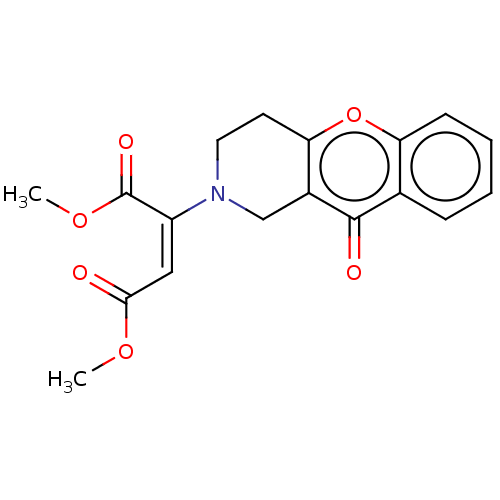 (CHEMBL4248994) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463668 (CHEMBL4242675) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463664 (CHEMBL4250143) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463671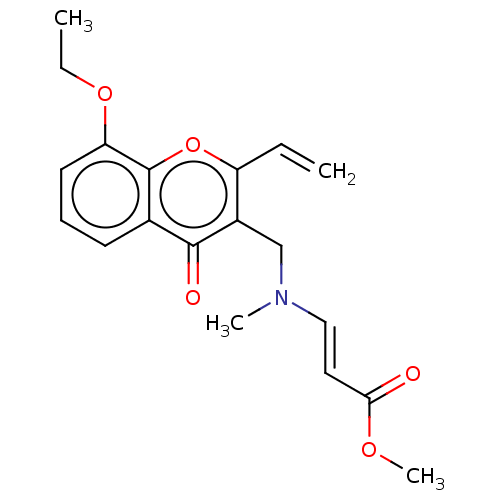 (CHEMBL4239718) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463672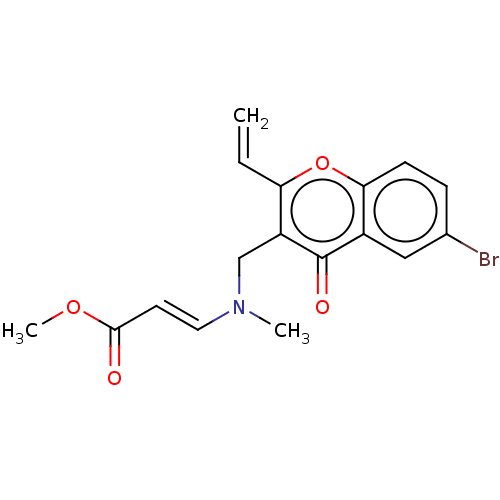 (CHEMBL4249829) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463673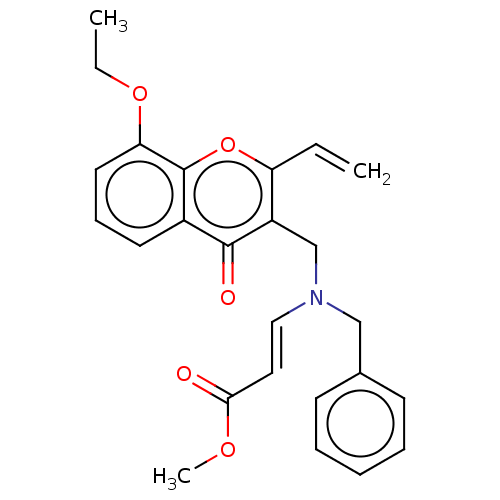 (CHEMBL4241628) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463665 (CHEMBL4240365) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463669 (CHEMBL4250510) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463674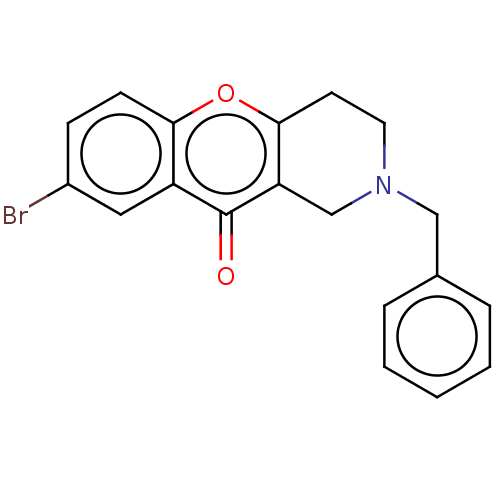 (CHEMBL4250950) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463660 (CHEMBL4250314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463661 (CHEMBL4238512) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463672 (CHEMBL4249829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463665 (CHEMBL4240365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463667 (CHEMBL4248378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463675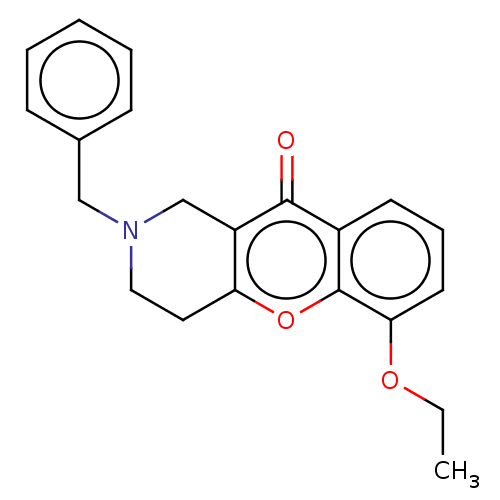 (CHEMBL4241100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50463674 (CHEMBL4250950) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's met... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463658 (CHEMBL4246032) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463660 (CHEMBL4250314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463670 (CHEMBL4248994) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463663 (CHEMBL4244573) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50463662 (CHEMBL4237467) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate incubated for 10 mins followed by substrate addition by spectro... | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463672 (CHEMBL4249829) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463673 (CHEMBL4241628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463657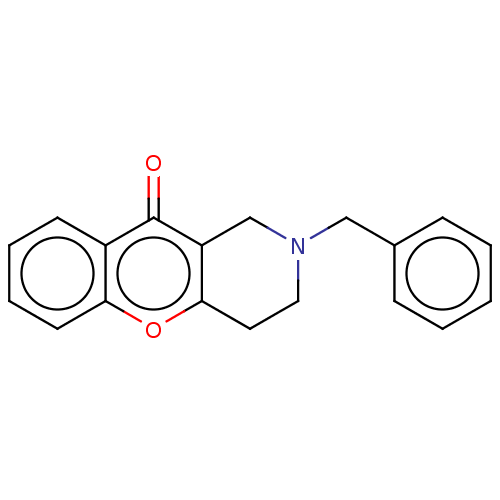 (CHEMBL4244773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463675 (CHEMBL4241100) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50463674 (CHEMBL4250950) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine iodide as substrate incubated for 10 mins followed by substrate addition by Ellman's method | Bioorg Med Chem 26: 4716-4725 (2018) Article DOI: 10.1016/j.bmc.2018.08.010 BindingDB Entry DOI: 10.7270/Q2RR21X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |