

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222244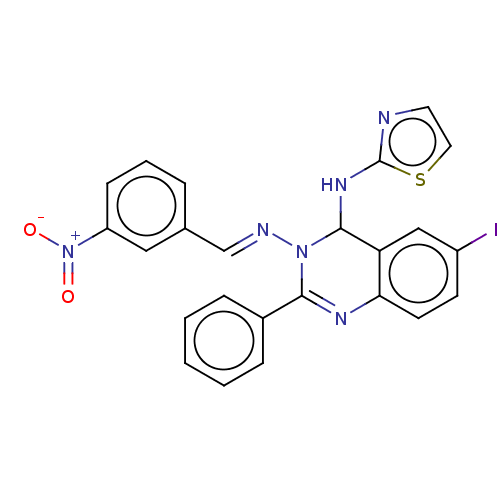 (6-Iodo-N3-(3-nitrobenzylidene)-2-phenyl-N4-(thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222254 (6-Iodo-N4-(4-methylthiazol-2-yl)-N3-(3-nitrobenzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222239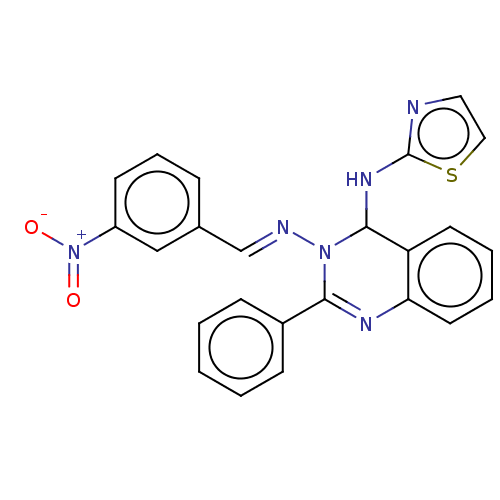 (N3-(3-Nitrobenzylidene)-2-phenyl-N4-(thiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222245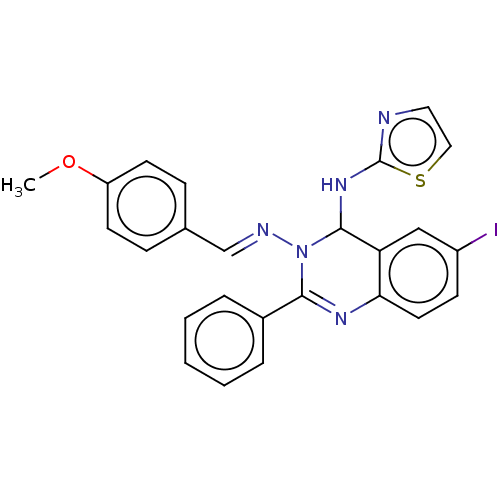 (6-Iodo-N3-(4-methoxybenzylidene)-2-phenyl-N4-(thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222255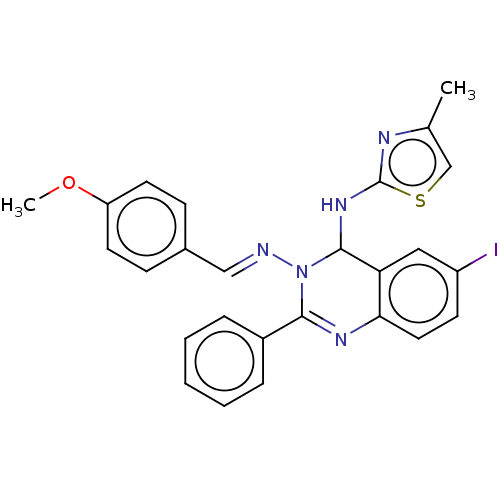 (6-Iodo-N3-(4-methoxybenzylidene)-N4-(4-methylthiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222249 (N4-(4-Methylthiazol-2-yl)-N3-(3-nitrobenzylidene)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222250 (N3-(4-methoxybenzylidene)-N4-(4-methylthiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222240 (N3-(4-Methoxybenzylidene)-2-phenyl-N4-(thiazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222247 (6-Iodo-N3-(2-methoxybenzylidene)-2-phenyl-N4-(thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50210628 (CHEMBL3946861) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of human PDE3A using fluorescein-labelled cAMP as substrate measured after 60 mins by IMAP TR-FRET assay | Eur J Med Chem 125: 143-189 (2017) Article DOI: 10.1016/j.ejmech.2016.09.023 BindingDB Entry DOI: 10.7270/Q23T9K6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222257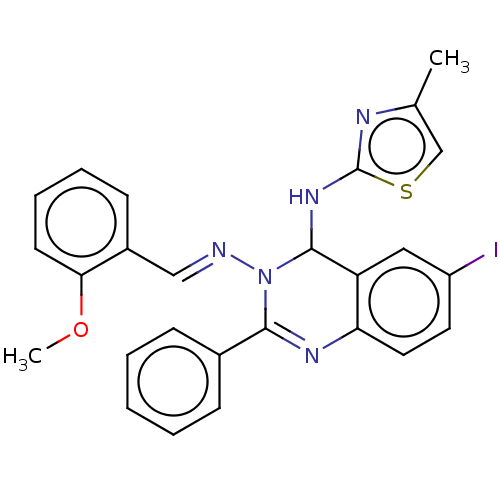 (6-Iodo-N3-(2-methoxybenzylidene)-N4-(4-methylthiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222252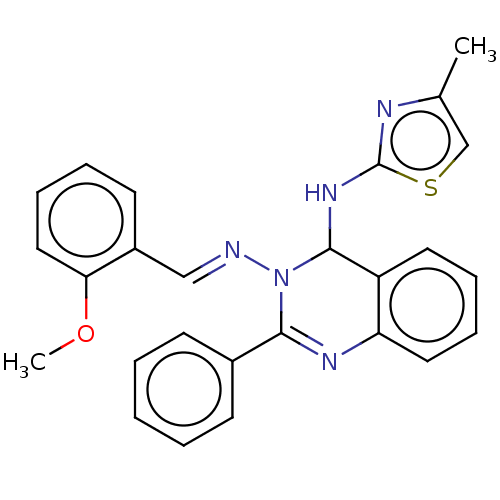 (N3-(2-methoxybenzylidene)-N4-(4-methylthiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222242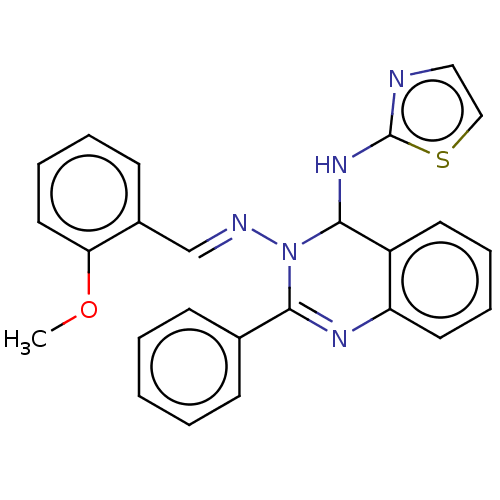 (N3-(2-Methoxybenzylidene)-2-phenyl-N4-(thiazol-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222256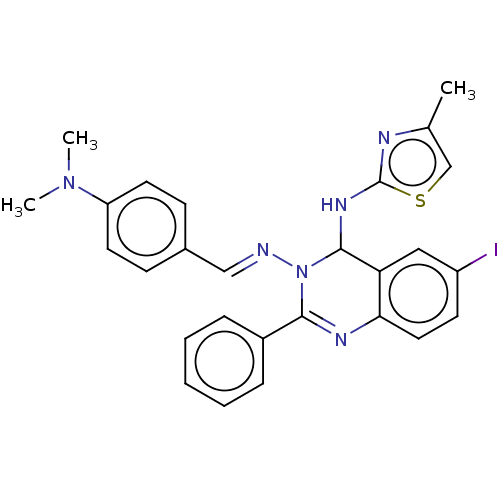 (N3-(4-(Dimethylamino)-benzylidene)-6-iodo-N4-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222251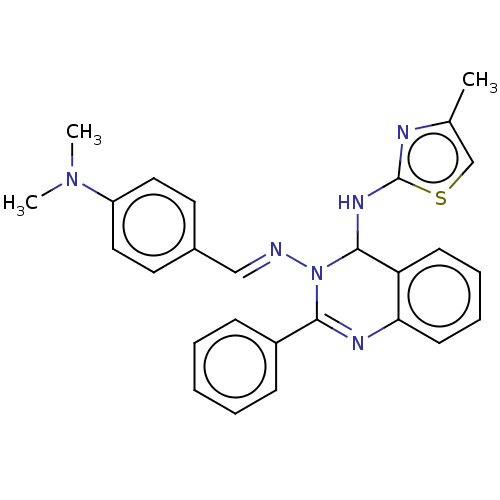 (N3-(4-(Dimethylamino)-benzylidene)-N4-(4-methylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222253 (N3-Benzylidene-6-iodo-N4-(4-methylthiazol-2-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222246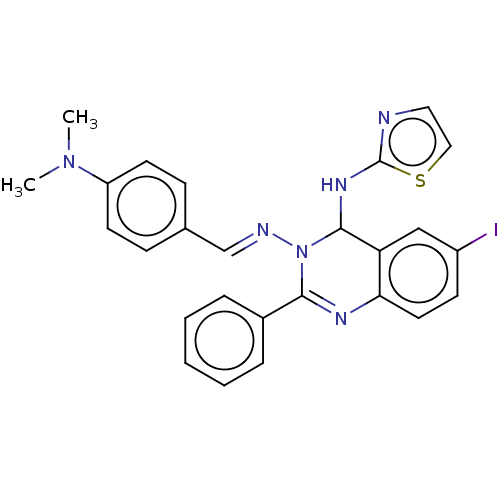 (N3-{4-(Dimethylamino)-benzylidene}-6-iodo-2-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222248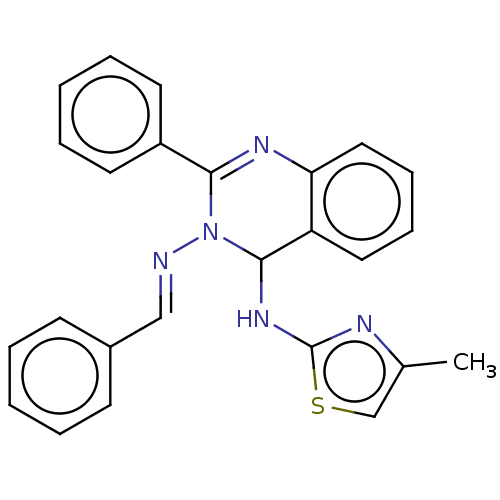 (N3-Benzylidene-N4-(4-methylthiazol-2-yl)-2-phenylq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222241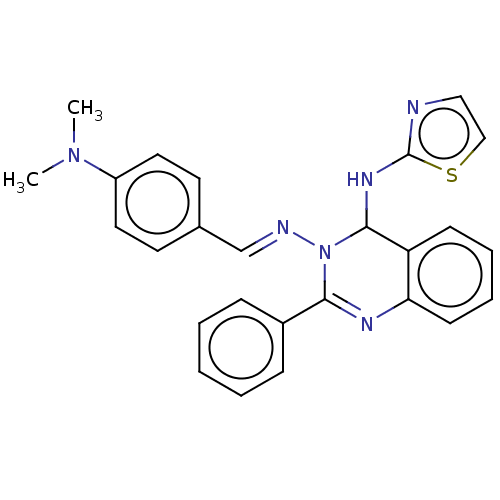 (N3-(4-(Dimethylamino)-benzylidene)-2-phenyl-N4-(th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222243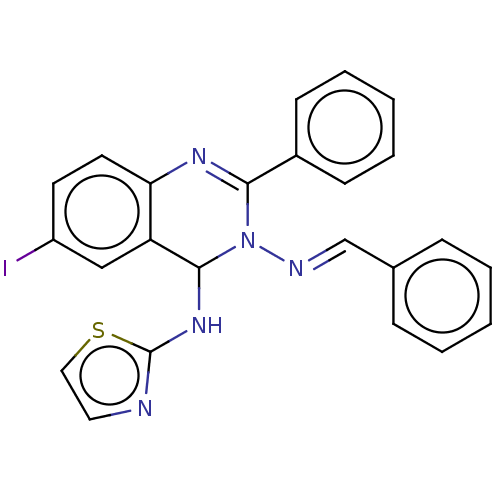 (N3-Benzylidene-6-iodo-2-phenyl-N4-(thiazol-2-yl)-q...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM222238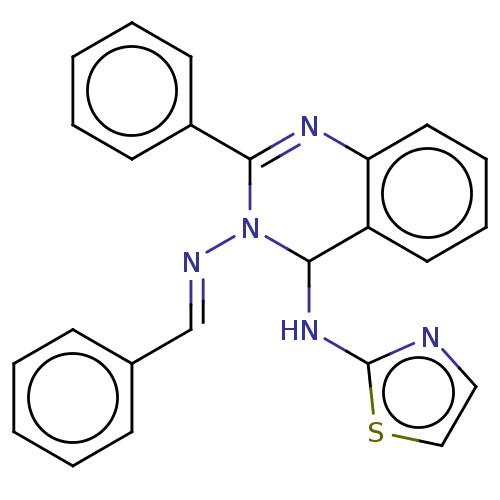 (N3-Benzylidene-2-phenyl-N4-(thiazol-2-yl)-quinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) | Assay Description The synthetic title compounds 7 (a-j) and 8 (a-j) were screened for in vitro DPP-4 inhibition using DPP-4 activity assay kit (Krishgen BioSystems). D... | Bioorg Chem 71: 181-191 (2017) Article DOI: 10.1016/j.bioorg.2017.02.004 BindingDB Entry DOI: 10.7270/Q2PR7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of HRG stimulated erbB2 phosphorylation in human MCF-7 cells preincubated for 90 mins followed by HRG addition for 5 mins by ELISA method | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232320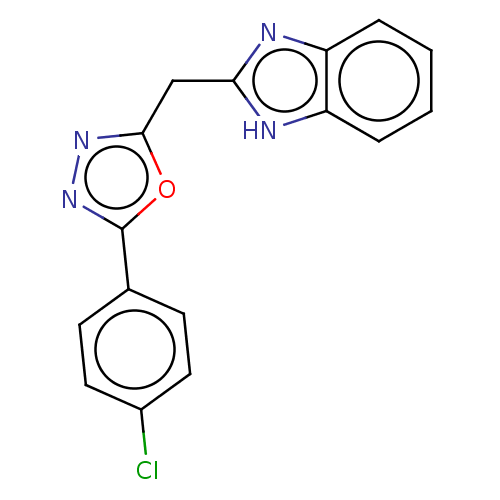 (CHEMBL4103370) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232320 (CHEMBL4103370) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232327 (CHEMBL4084249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232327 (CHEMBL4084249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50232320 (CHEMBL4103370) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of HRG stimulated erbB2 phosphorylation in human MCF-7 cells preincubated for 90 mins followed by HRG addition for 5 mins by ELISA method | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582491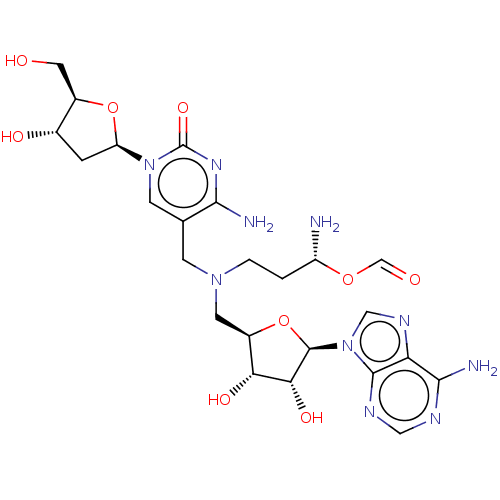 (US11518779, Compound 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582494 (US11518779, Compound 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50232327 (CHEMBL4084249) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of HRG stimulated erbB2 phosphorylation in human MCF-7 cells preincubated for 90 mins followed by HRG addition for 5 mins by ELISA method | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232328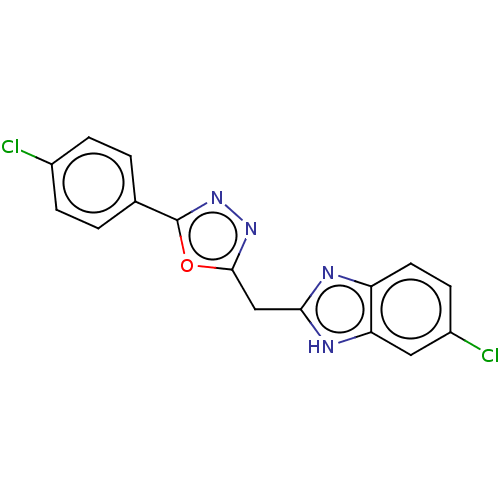 (CHEMBL4084996) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 979 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232328 (CHEMBL4084996) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582492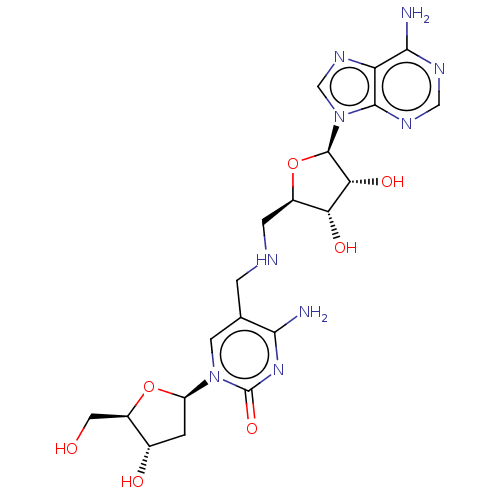 (US11518779, Compound 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173607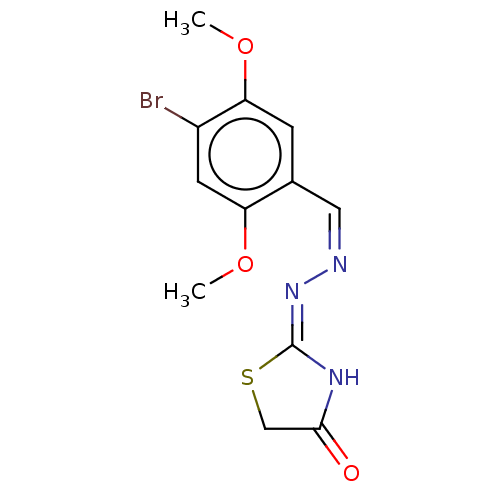 ((Z)-2-((Z)-(Napthalen-1-ylmethylene)hydrazono)thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582496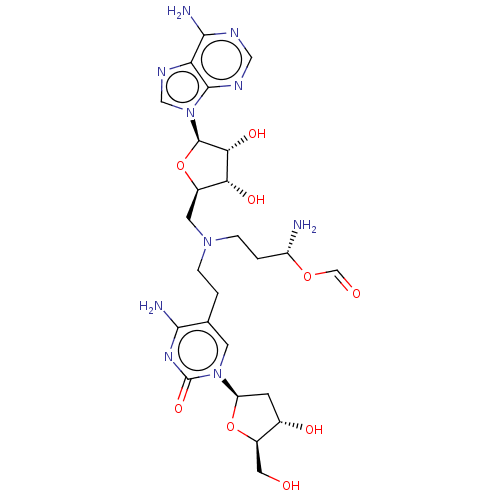 (US11518779, Compound 56) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582497 (US11518779, Compound 70) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173602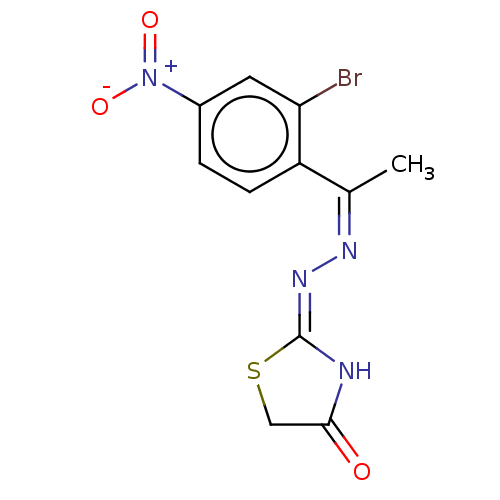 ((Z)-2-((Z)-(1-(2-Bromo-4-nitrophenyl)ethylidene)hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50232328 (CHEMBL4084996) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of HRG stimulated erbB2 phosphorylation in human MCF-7 cells preincubated for 90 mins followed by HRG addition for 5 mins by ELISA method | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582493 (US11518779, Compound 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232321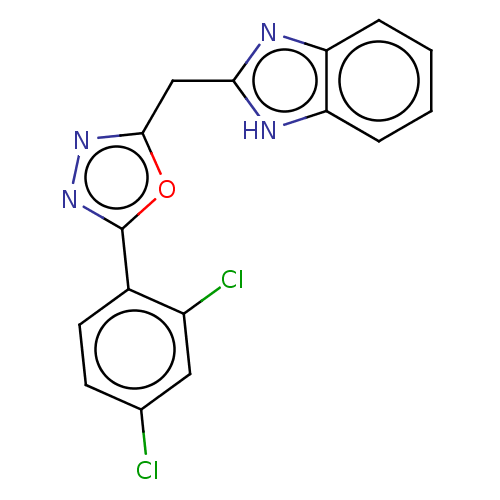 (CHEMBL4079125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232321 (CHEMBL4079125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232324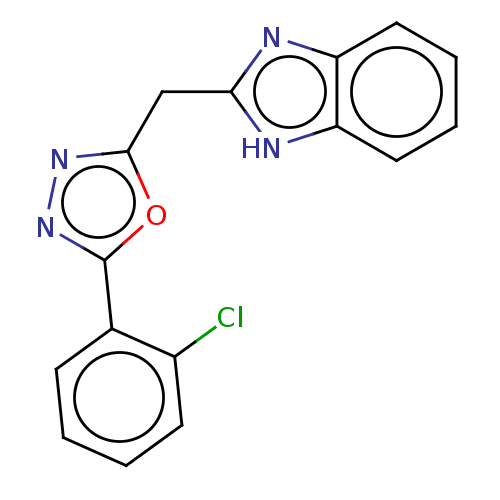 (CHEMBL4094471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232324 (CHEMBL4094471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50232321 (CHEMBL4079125) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of HRG stimulated erbB2 phosphorylation in human MCF-7 cells preincubated for 90 mins followed by HRG addition for 5 mins by ELISA method | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232344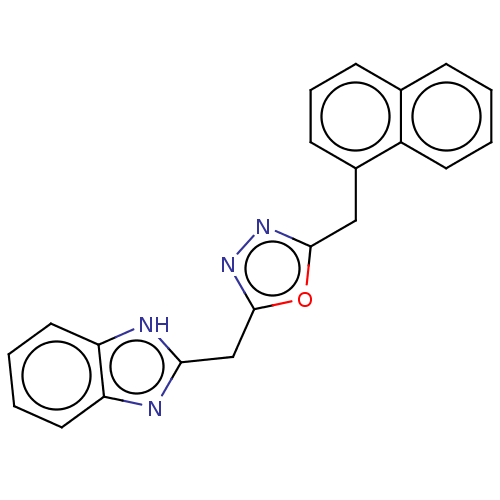 (CHEMBL4066118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50232344 (CHEMBL4066118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hamdard University Curated by ChEMBL | Assay Description Inhibition of EGF induced EGFR phosphorylation in human KB cells preincubated for 90 mins followed by EGF addition for 5 mins by sandwich ELISA metho... | Eur J Med Chem 126: 853-869 (2017) Article DOI: 10.1016/j.ejmech.2016.12.014 BindingDB Entry DOI: 10.7270/Q2CR5WKW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha (Bacillus pasteurii) | BDBM173604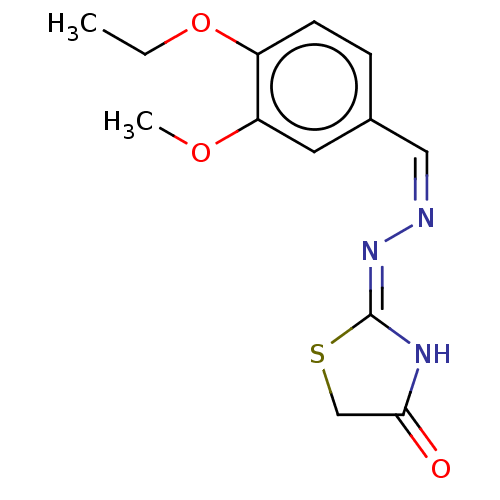 ((Z)-2-((Z)-(4-Ethoxy-3-methoxybenzoylidine)hydrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Hazara University | Assay Description This assay was modified from Berthelot assay and was employed for the determination of urease activity. The assay is based on the hydrolysis of urea ... | Bioorg Chem 63: 123-31 (2015) Article DOI: 10.1016/j.bioorg.2015.10.005 BindingDB Entry DOI: 10.7270/Q2H130RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM582498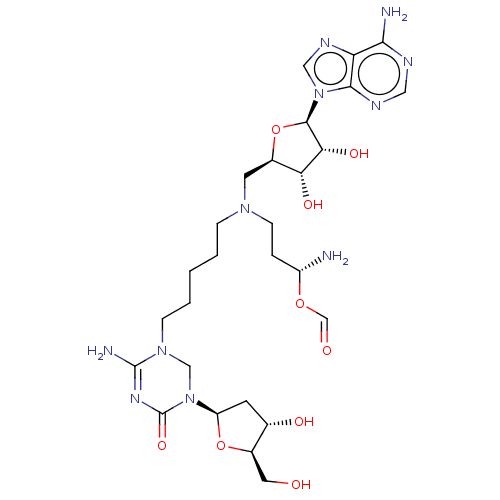 (US11518779, Compound 86) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P55SBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 125 total ) | Next | Last >> |