

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Piau£ Curated by ChEMBL | Assay Description Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assay | Eur J Med Chem 139: 401-411 (2017) Article DOI: 10.1016/j.ejmech.2017.08.019 BindingDB Entry DOI: 10.7270/Q2T43WMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50030474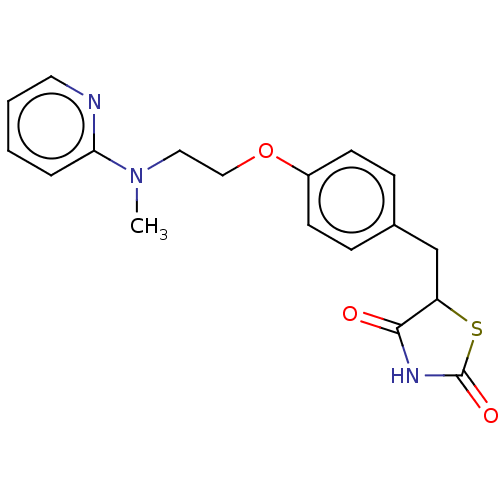 (Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM53804 (4-({2-[(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50387185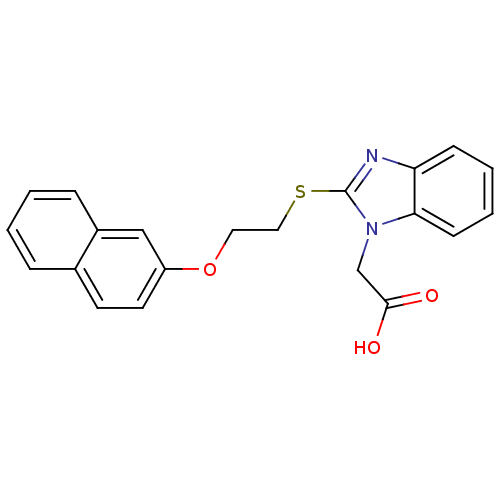 (CHEMBL2048021) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50493700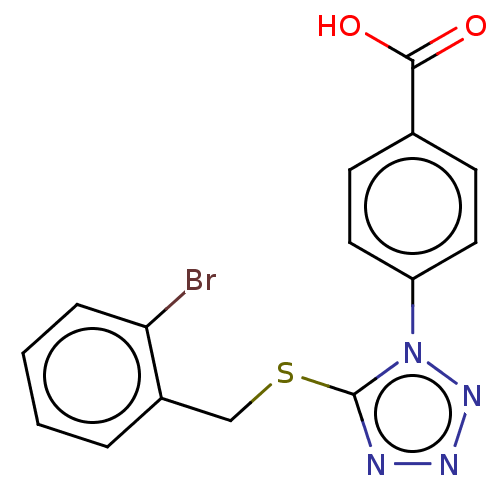 (CHEMBL1426715) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50277167 (CHEMBL4167962) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Piau£ Curated by ChEMBL | Assay Description Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assay | Eur J Med Chem 139: 401-411 (2017) Article DOI: 10.1016/j.ejmech.2017.08.019 BindingDB Entry DOI: 10.7270/Q2T43WMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50277166 (CHEMBL4163400) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Piau£ Curated by ChEMBL | Assay Description Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assay | Eur J Med Chem 139: 401-411 (2017) Article DOI: 10.1016/j.ejmech.2017.08.019 BindingDB Entry DOI: 10.7270/Q2T43WMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50277168 (CHEMBL4171298) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Piau£ Curated by ChEMBL | Assay Description Inhibition of Wistar rat plasma angiotensin 1-converting enzyme using H-hippuryl-His-Leu-OH as substrate after 20 mins by fluorescence assay | Eur J Med Chem 139: 401-411 (2017) Article DOI: 10.1016/j.ejmech.2017.08.019 BindingDB Entry DOI: 10.7270/Q2T43WMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||