 Found 491 hits with Last Name = 'gerasyuto' and Initial = 'ai'
Found 491 hits with Last Name = 'gerasyuto' and Initial = 'ai' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567964

(CHEMBL4858336 | US11903936, Compound 29)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cc(C)on1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567964

(CHEMBL4858336 | US11903936, Compound 29)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cc(C)on1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50365230

(CHEMBL1956285 | US11903936, Compound DSM265 | US92...)Show SMILES Cc1cc(Nc2ccc(cc2)S(F)(F)(F)(F)F)n2nc(nc2n1)C(C)(F)F Show InChI InChI=1S/C14H12F7N5S/c1-8-7-11(26-13(22-8)24-12(25-26)14(2,15)16)23-9-3-5-10(6-4-9)27(17,18,19,20)21/h3-7,23H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567990

(CHEMBL4846297 | US11903936, Compound 8)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1cn[nH]c1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567990

(CHEMBL4846297 | US11903936, Compound 8)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567992
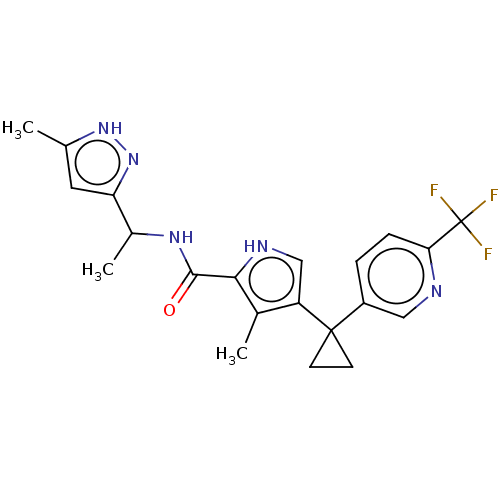
(CHEMBL4846204 | US11903936, Compound 11)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1cc(C)[nH]n1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567995
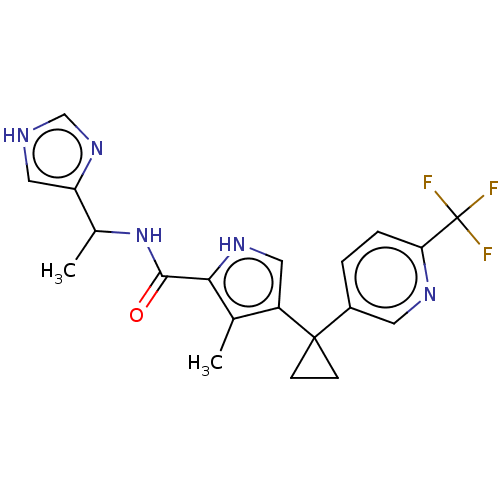
(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM651975

(3-methyl-N-(1-(1H-pyrazol-4-yl)ethyl)-4-(((trifluo...)Show SMILES CC(NC(=O)c1[nH]cc(Cc2cccnc2C(F)(F)F)c1C)c1cn[nH]c1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567994

(CHEMBL4864218 | US11903936, Compound 17)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1cc([nH]n1)C#N | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM651975

(3-methyl-N-(1-(1H-pyrazol-4-yl)ethyl)-4-(((trifluo...)Show SMILES CC(NC(=O)c1[nH]cc(Cc2cccnc2C(F)(F)F)c1C)c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567967

(CHEMBL4850393 | US11903936, Compound 35)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(nc2)C(F)(F)F)c1C)c1cnn(C)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567992

(CHEMBL4846204 | US11903936, Compound 11)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1cc(C)[nH]n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM651960

(N-(1-(1H-1,2,4-triazol-3-yl) ethyl)-4-(3-fluoro-4-...)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(c(F)c2)C(F)(F)F)c1C)c1nc[nH]n1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50365230

(CHEMBL1956285 | US11903936, Compound DSM265 | US92...)Show SMILES Cc1cc(Nc2ccc(cc2)S(F)(F)(F)(F)F)n2nc(nc2n1)C(C)(F)F Show InChI InChI=1S/C14H12F7N5S/c1-8-7-11(26-13(22-8)24-12(25-26)14(2,15)16)23-9-3-5-10(6-4-9)27(17,18,19,20)21/h3-7,23H,1-2H3 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM50567998
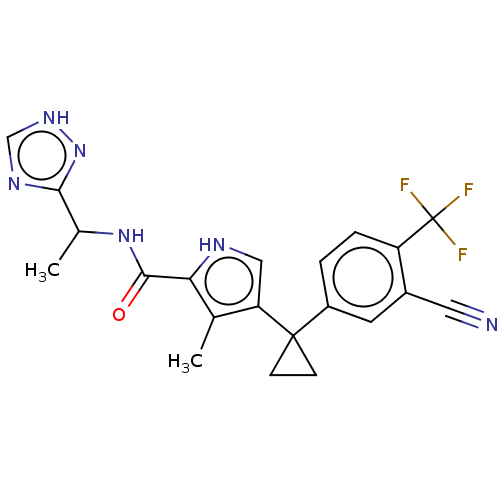
(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567995

(CHEMBL4851471 | US11903936, Compound 20)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1c[nH]cn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567994

(CHEMBL4864218 | US11903936, Compound 17)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1cc([nH]n1)C#N | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567998

(CHEMBL4876457 | US11903936, Compound 26)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM50567991

(CHEMBL4848632 | US11903936, Compound 2)Show SMILES CC(NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(nc1)C(F)(F)F)c1nc[nH]n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM651960

(N-(1-(1H-1,2,4-triazol-3-yl) ethyl)-4-(3-fluoro-4-...)Show SMILES CC(NC(=O)c1[nH]cc(Cc2ccc(c(F)c2)C(F)(F)F)c1C)c1nc[nH]n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM651971
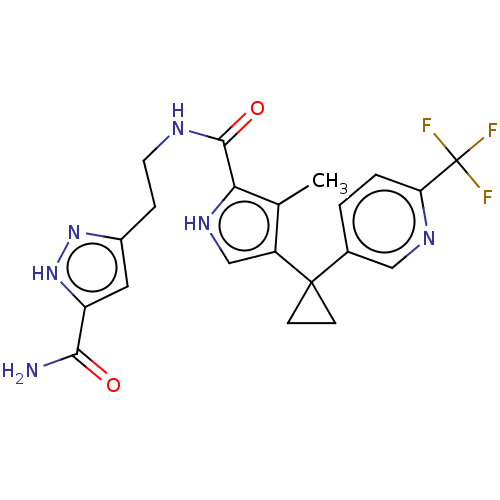
(3-((1-(3-methyl-4-(1-(6-(trifluoromethyl)pyridin-3...)Show SMILES Cc1c(c[nH]c1C(=O)NCCc1cc([nH]n1)C(N)=O)C1(CC1)c1ccc(nc1)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Plasmodium falciparum (isolate 3D7)) | BDBM651979

(US11903936, Compound 25)Show SMILES C[C@H](NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM651971

(3-((1-(3-methyl-4-(1-(6-(trifluoromethyl)pyridin-3...)Show SMILES Cc1c(c[nH]c1C(=O)NCCc1cc([nH]n1)C(N)=O)C1(CC1)c1ccc(nc1)C(F)(F)F | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(malaria parasite P. vivax) | BDBM651979

(US11903936, Compound 25)Show SMILES C[C@H](NC(=O)c1[nH]cc(c1C)C1(CC1)c1ccc(c(c1)C#N)C(F)(F)F)c1nc[nH]n1 |r| | MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM602728

(2-[1-(2-Isoindolin-2-yl-6-methyl-4-oxo-chromen-8-y...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2N58RBV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602702
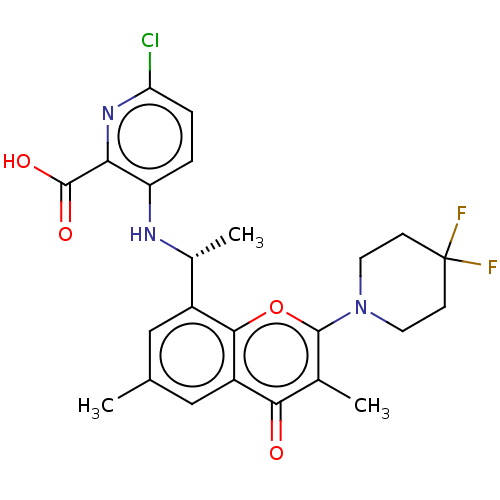
(US11649227, Example 310 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccc(Cl)nc1C(O)=O)c1cc(C)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602703

(US11649227, Example 319 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602704
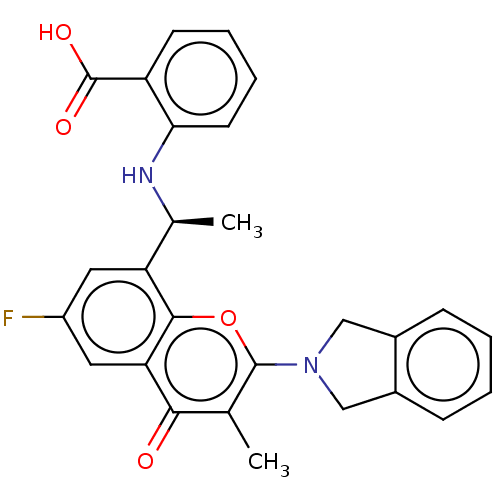
(2-[1-(6-Fluoro-2-isoindolin-2-yl-3-methyl-4-oxo-ch...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602704

(2-[1-(6-Fluoro-2-isoindolin-2-yl-3-methyl-4-oxo-ch...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602705

(2-[1-[2-(4,4-Difluoro-1-piperidyl)-6-fluoro-3-meth...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602705

(2-[1-[2-(4,4-Difluoro-1-piperidyl)-6-fluoro-3-meth...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC(F)(F)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602706

(2-[1-[2-(6-Azaspiro[2.5]octan-6-yl)-6-fluoro-3-met...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602706

(2-[1-[2-(6-Azaspiro[2.5]octan-6-yl)-6-fluoro-3-met...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(N1CCC3(CC3)CC1)c(C)c2=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602707
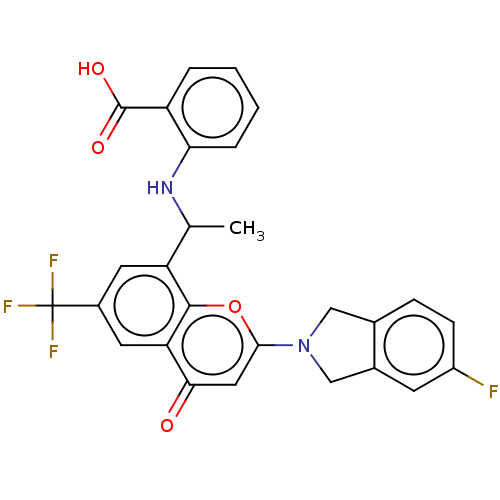
(US11649227, Example 335 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602708

(2-[1-[2-(5-Cyanoisoindolin-2- yl)-4-oxo-6- (triflu...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccc(cc2C1)C#N)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602708

(2-[1-[2-(5-Cyanoisoindolin-2- yl)-4-oxo-6- (triflu...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccc(cc2C1)C#N)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602709

(2-[1-[2-Isoindolin-2-yl-4-oxo-6-(trifluoromethyl)c...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccccc2C1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602709

(2-[1-[2-Isoindolin-2-yl-4-oxo-6-(trifluoromethyl)c...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1Cc2ccccc2C1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602710
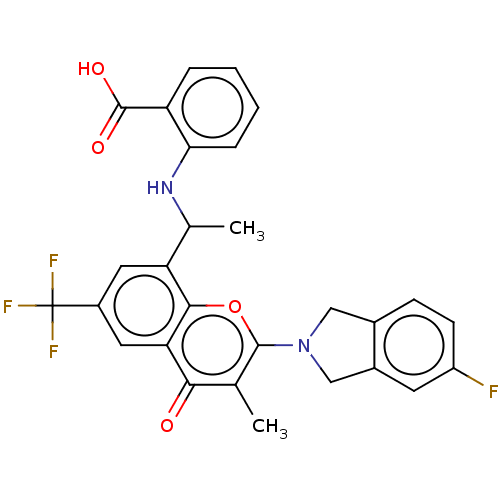
(US11649227, Example 343 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(N1Cc3ccc(F)cc3C1)c(C)c2=O)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602711

(2-[1-[2-Isoindolin-2-yl-3-methyl-4-oxo-6-(trifluor...)Show SMILES C[C@H](Nc1ccccc1C(O)=O)c1cc(cc2c1oc(N1Cc3ccccc3C1)c(C)c2=O)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM602712
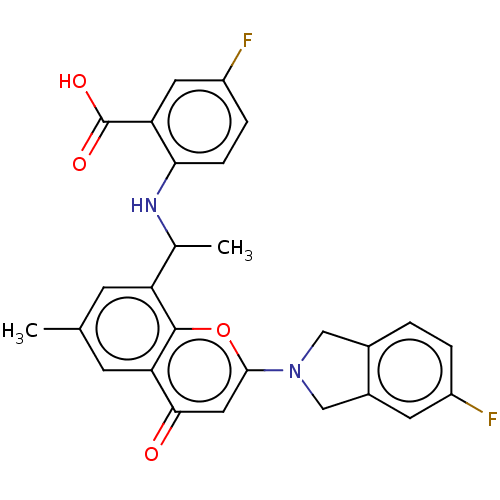
(US11649227, Example 349 | US20230286960, Example 3...)Show SMILES CC(Nc1ccc(F)cc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602712

(US11649227, Example 349 | US20230286960, Example 3...)Show SMILES CC(Nc1ccc(F)cc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccc(F)cc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602714
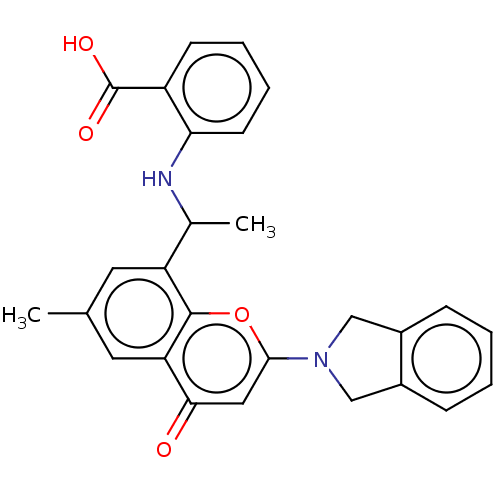
(US11649227, Example 362 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1Cc2ccccc2C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602715
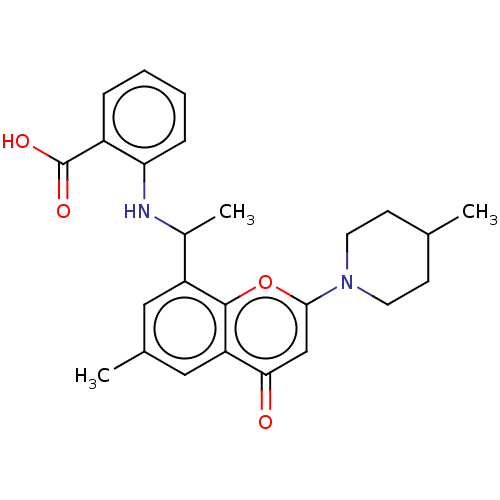
(US11649227, Example 370 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCC(C)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602718
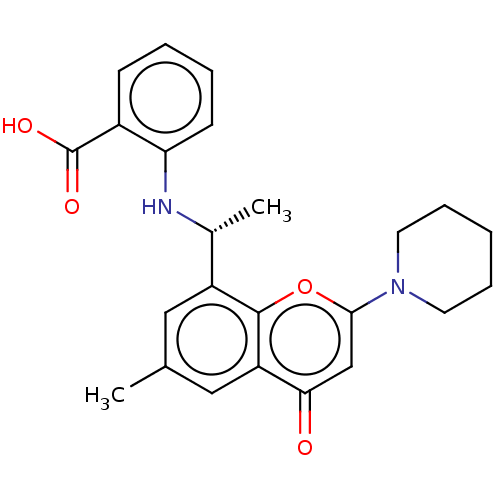
(US11649227, Example 376 | US20230286960, Example 3...)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cc2c1oc(cc2=O)N1CCCCC1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602723

(US11649227, Example 396 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(cc2c1oc(cc2=O)N1CCC(F)(F)CC1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform [H1047R]
(Homo sapiens (Human)) | BDBM602724
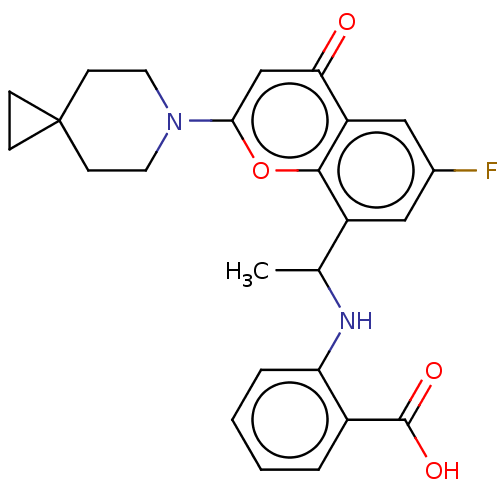
(US11649227, Example 398 | US20230286960, Example 3...)Show SMILES CC(Nc1ccccc1C(O)=O)c1cc(F)cc2c1oc(cc2=O)N1CCC2(CC2)CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28K7F74 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data