

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitinase (Onchocerca volvulus) | BDBM50063753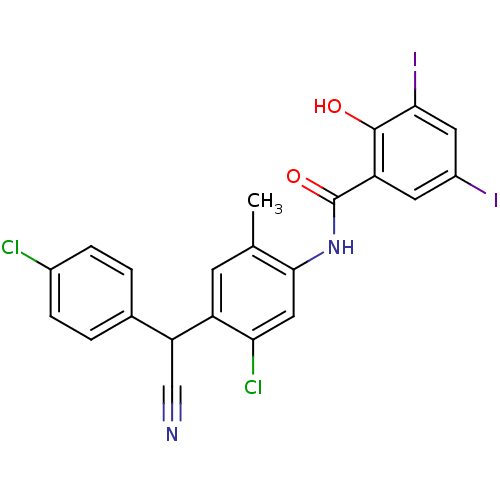 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM50565476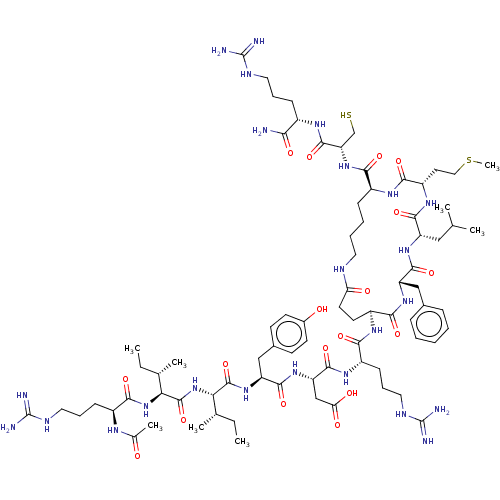 (CHEMBL4780117) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 10His-tagged eIF4E expressed in Escherichia coli BL21 (DE3) cells by catalytic enzyme-linked click chemistry assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112655 BindingDB Entry DOI: 10.7270/Q2KS6W8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM50565475 (CHEMBL4777401) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 10His-tagged eIF4E expressed in Escherichia coli BL21 (DE3) cells by catalytic enzyme-linked click chemistry assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112655 BindingDB Entry DOI: 10.7270/Q2KS6W8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM50525088 (CHEMBL4461497) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 10His-tagged eIF4E expressed in Escherichia coli BL21 (DE3) cells by catalytic enzyme-linked click chemistry assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112655 BindingDB Entry DOI: 10.7270/Q2KS6W8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347553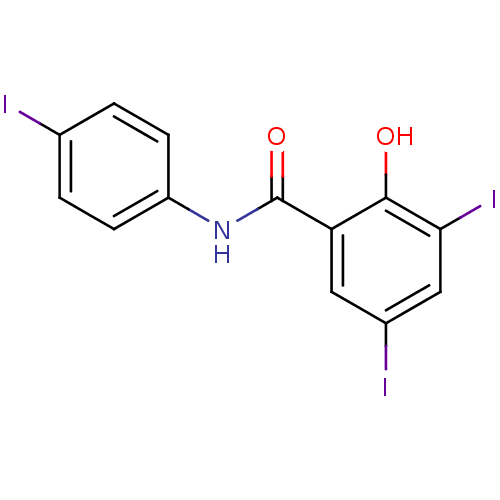 (CHEMBL1801794) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347552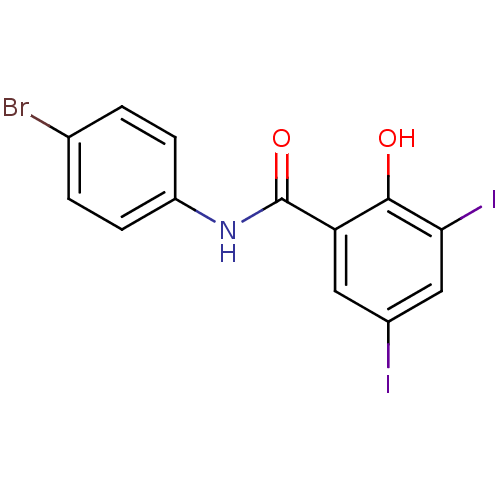 (CHEMBL391125) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347551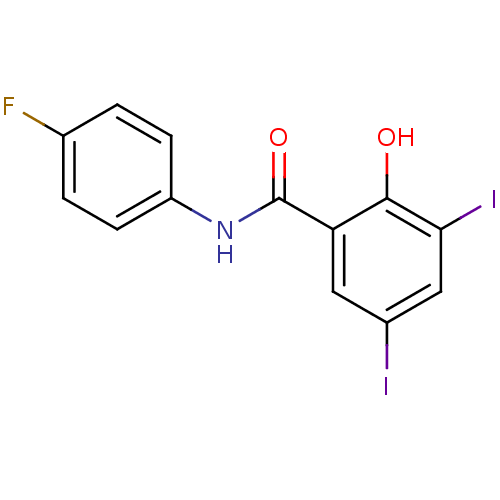 (CHEMBL395873) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347554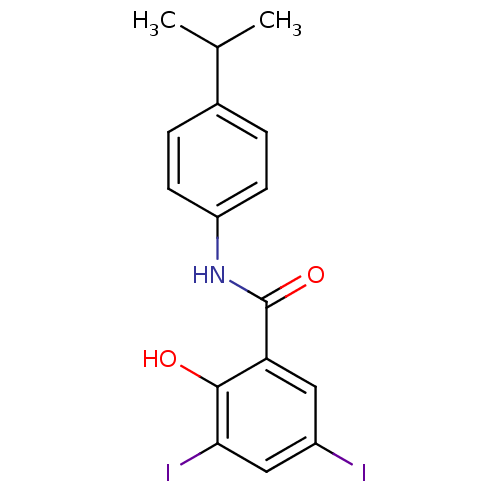 (CHEMBL1801795) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50063753 (CHEMBL12131 | Closantel | N-(5-chloro-4-((4-chloro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347549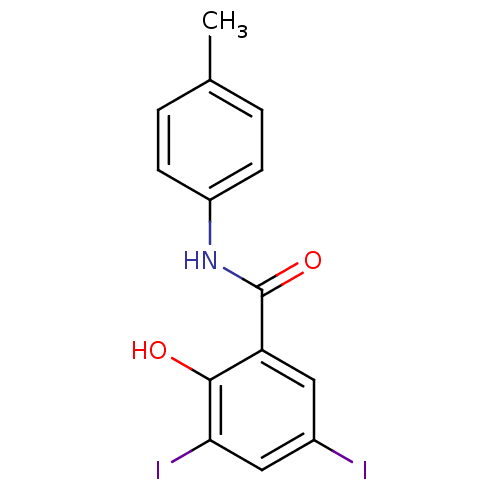 (CHEMBL482487) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50348824 (CHEMBL4177195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485657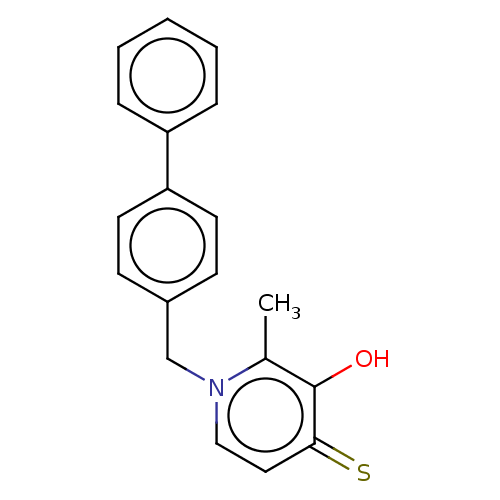 (CHEMBL2146996) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485648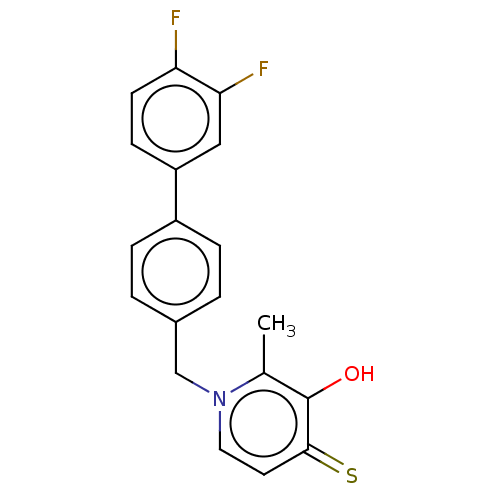 (CHEMBL2146995) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485651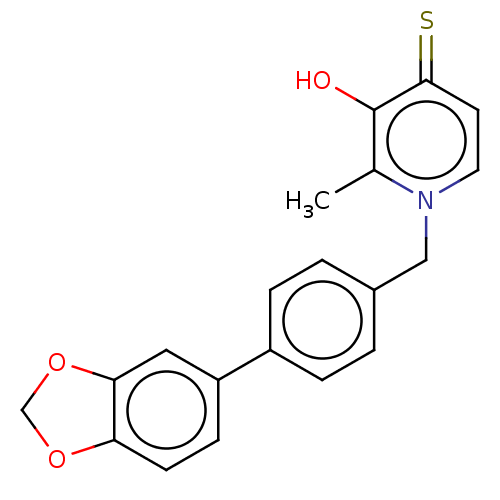 (CHEMBL2146997) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50348830 (CHEMBL4169355) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50342407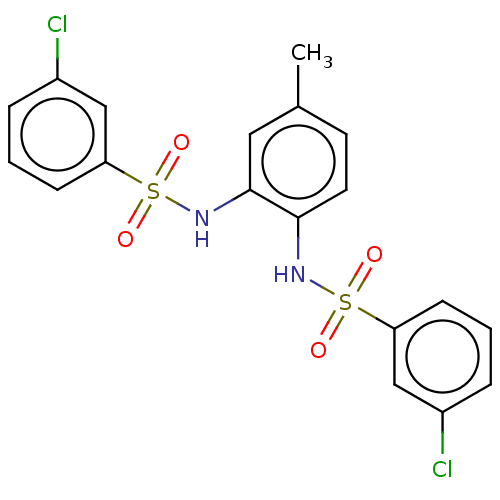 (CHEMBL4174649) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485646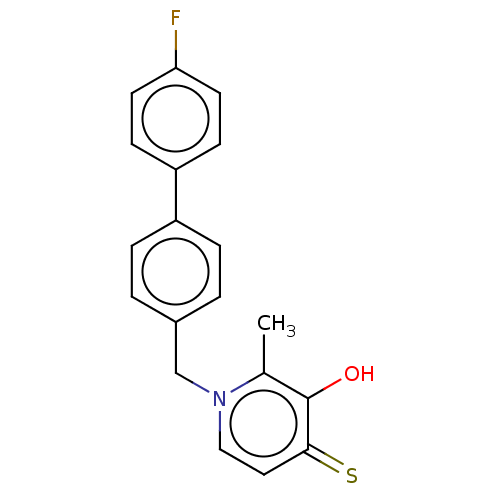 (CHEMBL2146998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485653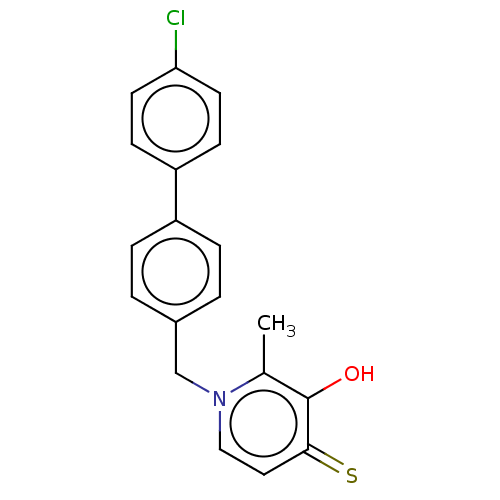 (CHEMBL2147001) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347555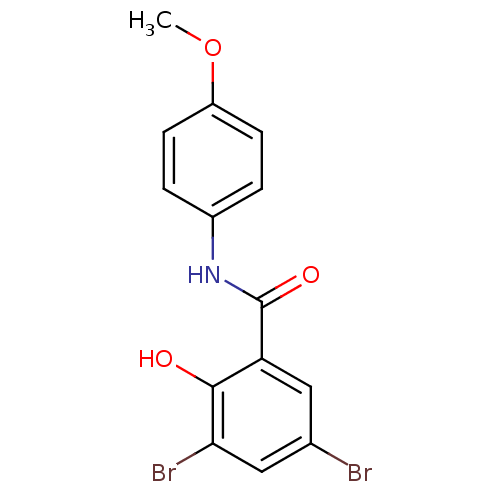 (3,5-Dibromo-2-hydroxy-N-(4-methoxyphenyl)benzamide...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50346949 (CHEMBL4163991) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347548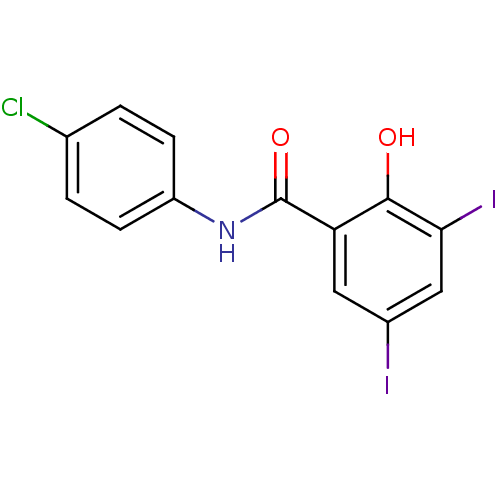 (CHEMBL237819) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485647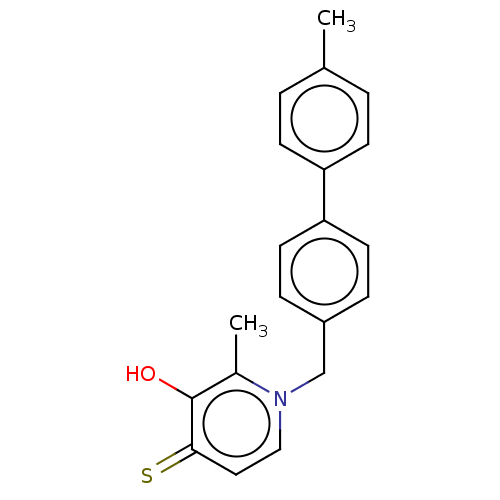 (CHEMBL2146999) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50342411 (CHEMBL4170052) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485654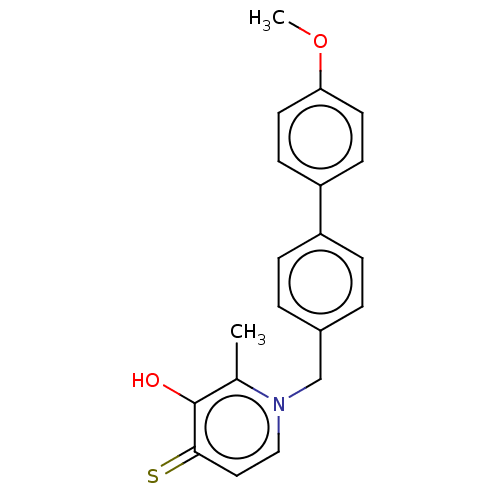 (CHEMBL2147000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50347550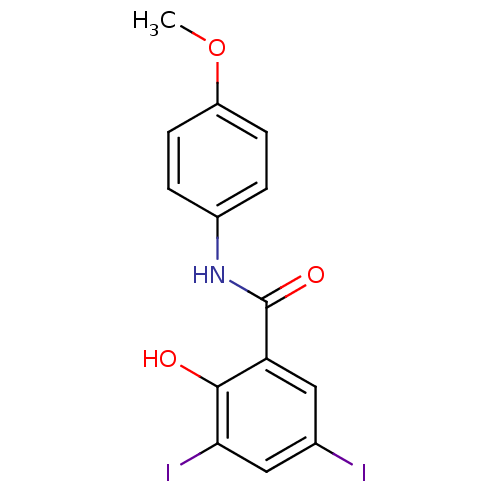 (CHEMBL484912) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50342408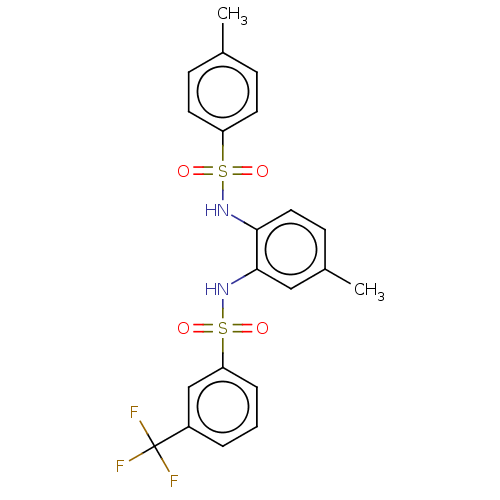 (CHEMBL4162127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50350508 (CHEMBL4165508) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345905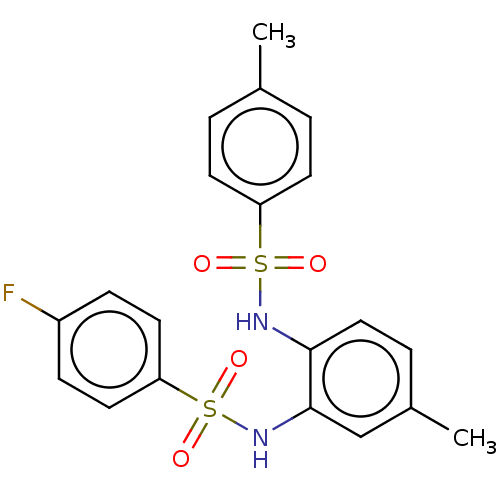 (CHEMBL4167853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Onchocerca volvulus) | BDBM50065988 (3,5-Dichloro-N-(4-chloro-phenyl)-2-hydroxy-benzami...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Skaggs Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of Onchocerca volvulus L3 larvae chitinase using 4-methylumbelliferyl-N,N',N''-beta-chitotrioside as a profluorescent substrate after 10 m... | J Med Chem 54: 3963-72 (2011) Article DOI: 10.1021/jm200364n BindingDB Entry DOI: 10.7270/Q21V5FB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50015094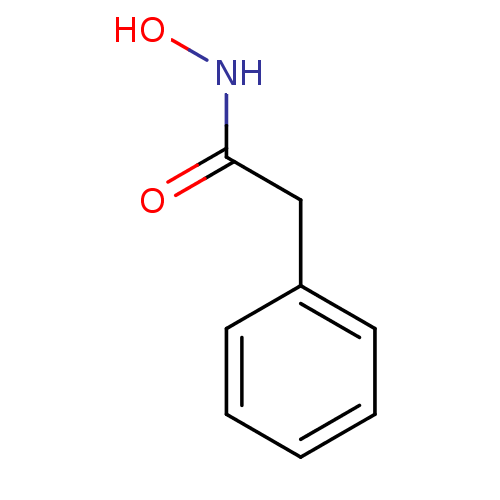 (CHEMBL152665 | N-Hydroxy-2-phenyl-acetamide | N-hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345479 (CHEMBL4172292) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345477 (CHEMBL4171210) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50350836 (CHEMBL4175708) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50342409 (CHEMBL4161003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485655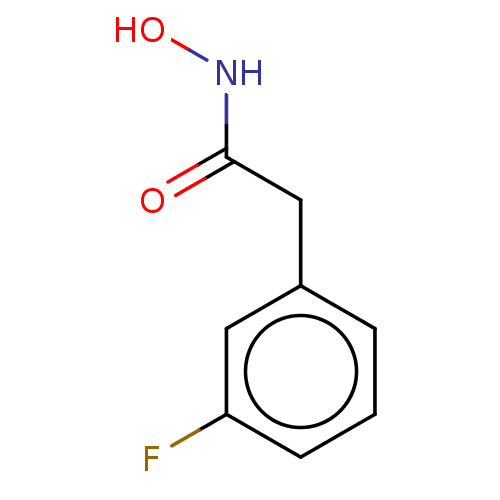 (CHEMBL2146994) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345903 (CHEMBL4167436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345902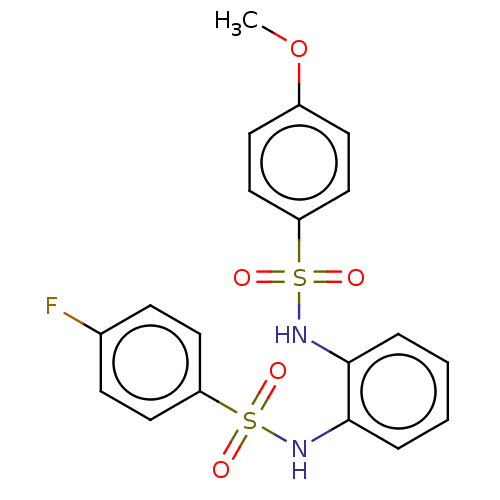 (CHEMBL4176121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50342410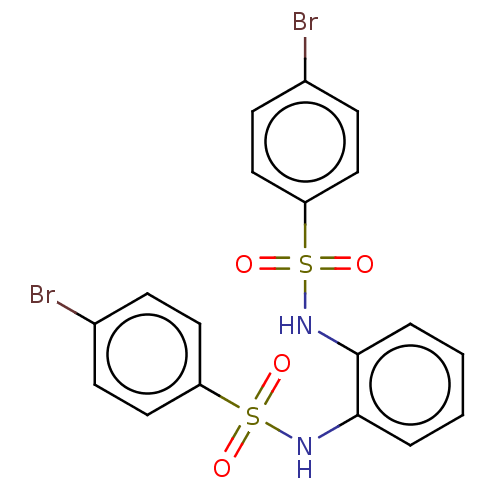 (CHEMBL4159534) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345475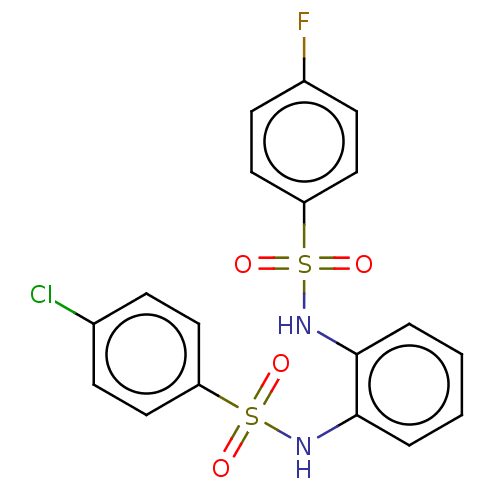 (CHEMBL4175704) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50350481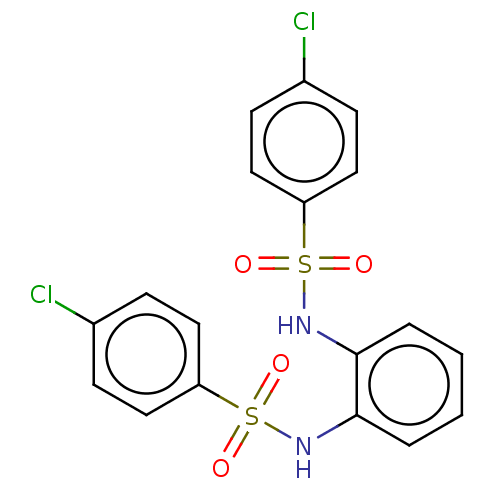 (CHEMBL4168929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345901 (CHEMBL4167849) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50346881 (CHEMBL4160599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345904 (CHEMBL2139421) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345478 (CHEMBL4164411) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein lin-28 homolog A (Mus musculus) | BDBM50345476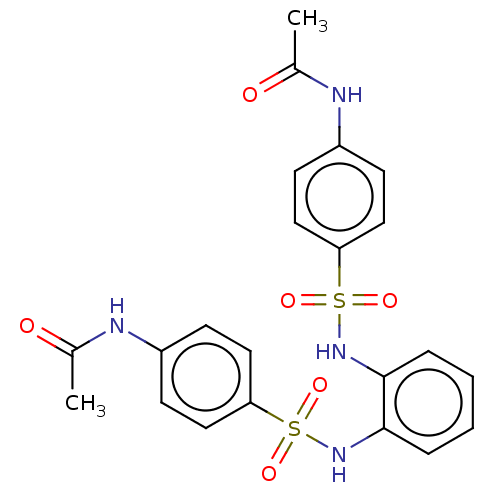 (CHEMBL4063466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to mouse N-terminal Halo-fused/biotin-labelled Lin28A expressed in Escherichia coli BL21(DE3) assessed as inhibition of protein inte... | ACS Med Chem Lett 9: 517-521 (2018) Article DOI: 10.1021/acsmedchemlett.8b00126 BindingDB Entry DOI: 10.7270/Q24T6MXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485649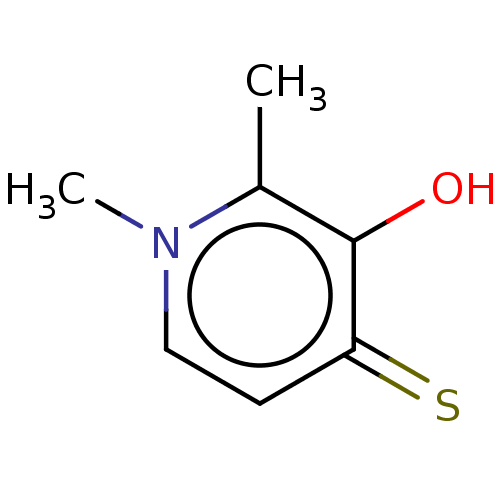 (CHEMBL1650620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485656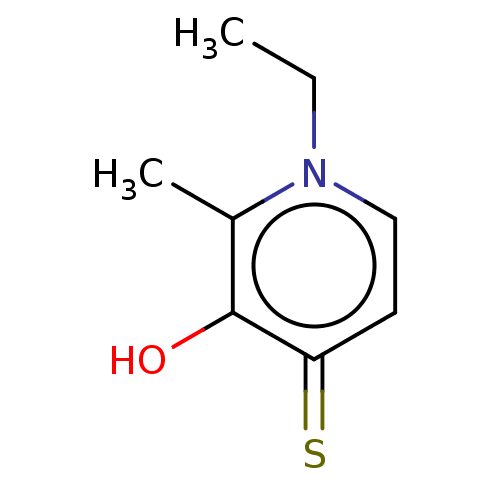 (CHEMBL1650624) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485652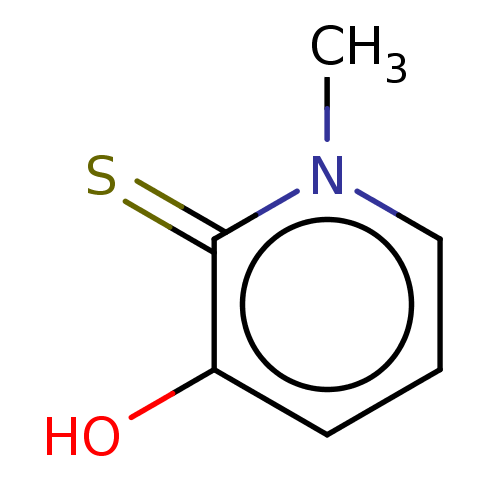 (CHEMBL1650621) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Elastase (Pseudomonas aeruginosa) | BDBM50485650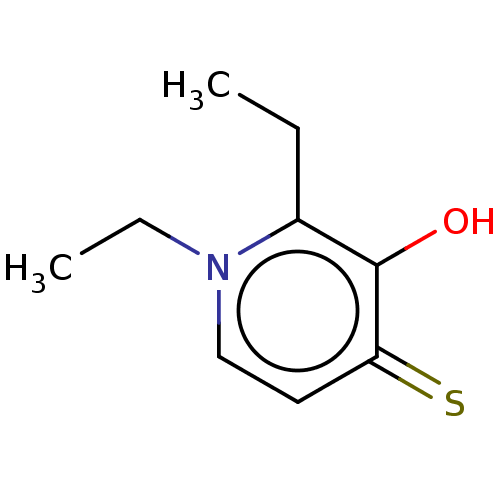 (CHEMBL1650623) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasB using Abz-Ala-Gly-Leu-Ala-p-Nitro-Benzyl-Amide as substrate incubated for 30 mins prior to substra... | ACS Med Chem Lett 3: 668-672 (2012) Article DOI: 10.1021/ml300128f BindingDB Entry DOI: 10.7270/Q2BG2RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM50525092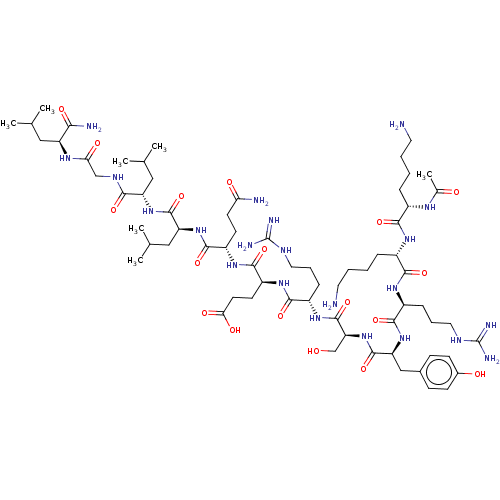 (CHEMBL4444144) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to N-terminal 10His-tagged m7GDP-bound recombinant human eIF4E expressed in Escherichia coli BL21 (DE3) cells assessed as dissociati... | J Med Chem 62: 4967-4978 (2019) Article DOI: 10.1021/acs.jmedchem.9b00068 BindingDB Entry DOI: 10.7270/Q2JQ14FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |