

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Bos taurus (bovine)) | BDBM21955 (4-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxym...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Maryland Baltimore County | Assay Description The target compounds were screened against calf intestine ADA in vitro by monitoring the rate of hydrolyzing adenosine into inosine spectrophotometri... | J Med Chem 51: 694-8 (2008) Article DOI: 10.1021/jm700931t BindingDB Entry DOI: 10.7270/Q20R9MP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM21953 (4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | -28.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Maryland Baltimore County | Assay Description The target compounds were screened against calf intestine ADA in vitro by monitoring the rate of hydrolyzing adenosine into inosine spectrophotometri... | J Med Chem 51: 694-8 (2008) Article DOI: 10.1021/jm700931t BindingDB Entry DOI: 10.7270/Q20R9MP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM21957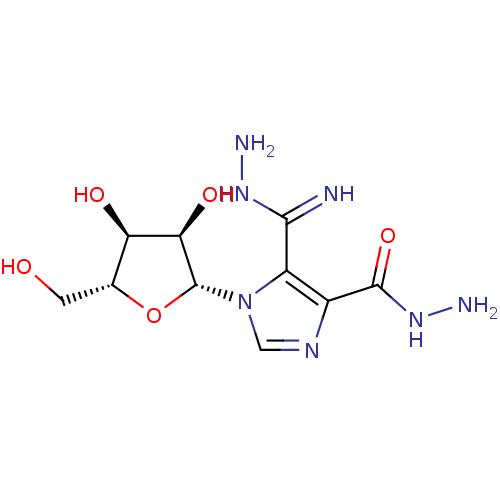 (4-(hydrazinocarbonyl)-1-beta-D-ribofuranosyl-1H-im...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.65E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Maryland Baltimore County | Assay Description The target compounds were screened against calf intestine ADA in vitro by monitoring the rate of hydrolyzing adenosine into inosine spectrophotometri... | J Med Chem 51: 694-8 (2008) Article DOI: 10.1021/jm700931t BindingDB Entry DOI: 10.7270/Q20R9MP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM21956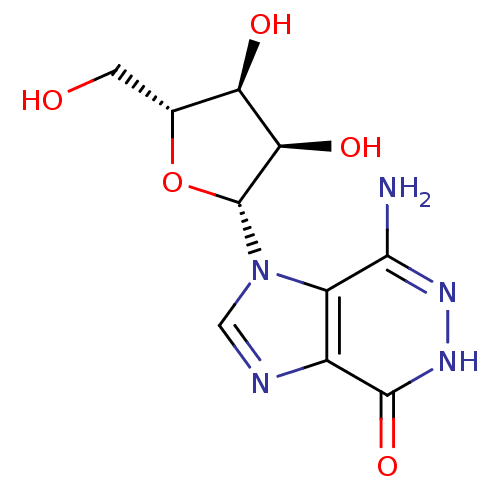 (7-amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxym...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.14E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Maryland Baltimore County | Assay Description The target compounds were screened against calf intestine ADA in vitro by monitoring the rate of hydrolyzing adenosine into inosine spectrophotometri... | J Med Chem 51: 694-8 (2008) Article DOI: 10.1021/jm700931t BindingDB Entry DOI: 10.7270/Q20R9MP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM21954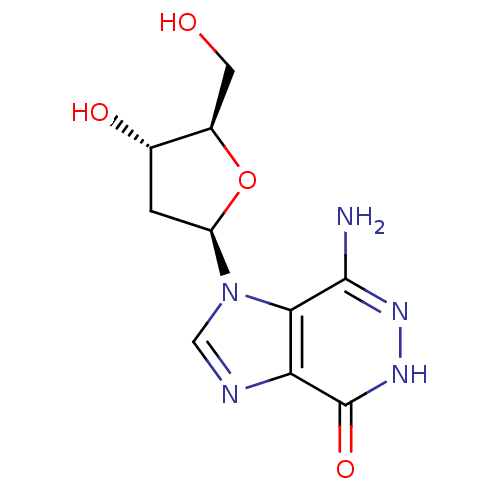 (7-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.20E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Maryland Baltimore County | Assay Description The target compounds were screened against calf intestine ADA in vitro by monitoring the rate of hydrolyzing adenosine into inosine spectrophotometri... | J Med Chem 51: 694-8 (2008) Article DOI: 10.1021/jm700931t BindingDB Entry DOI: 10.7270/Q20R9MP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50012278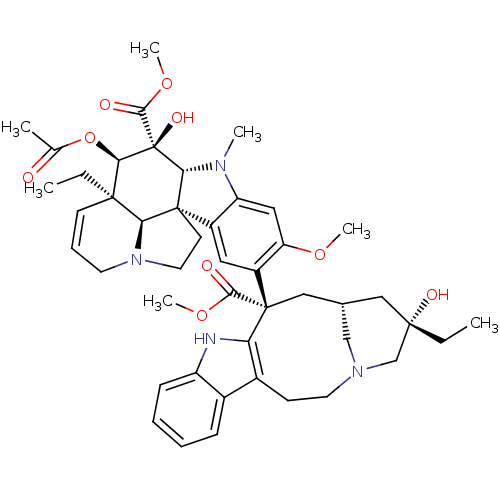 ((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474288 (CHEMBL112192) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to displace [3H]colchicine from its binding site on tubulin | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50035218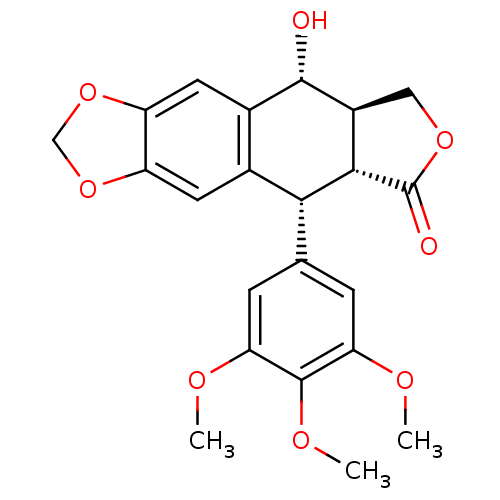 (CHEMBL61 | PODOFILOX | Podophyllinic acid lactone ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474300 (CHEMBL116173) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to displace [3H]colchicine from its binding site on Tubulin | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107672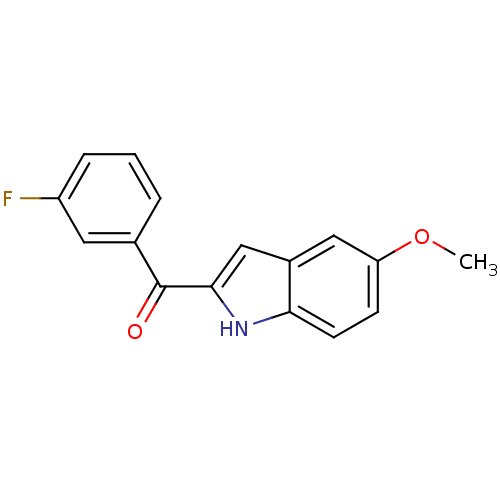 ((3-Fluoro-phenyl)-(5-methoxy-1H-indol-2-yl)-methan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474288 (CHEMBL112192) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474290 (CHEMBL115499) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107658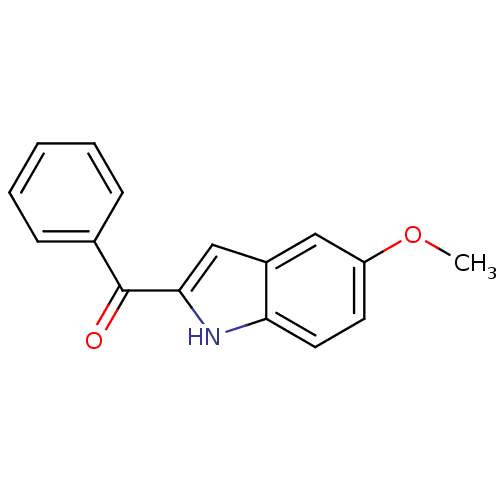 ((5-Methoxy-1H-indol-2-yl)-phenyl-methanone | (5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107678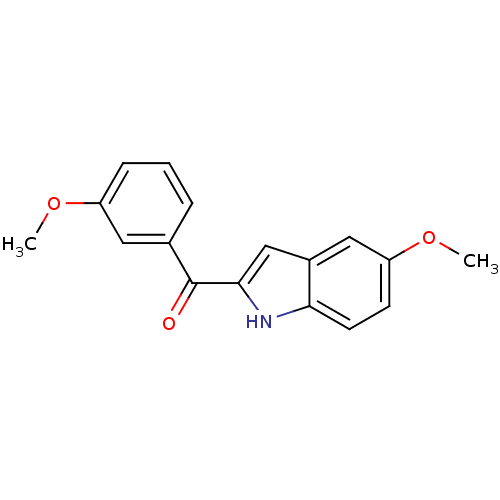 ((5-Methoxy-1H-indol-2-yl)-(3-methoxy-phenyl)-metha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474293 (CHEMBL324797) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474298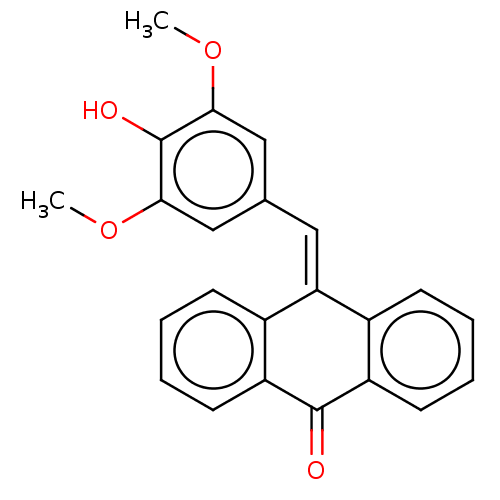 (CHEMBL321632) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107677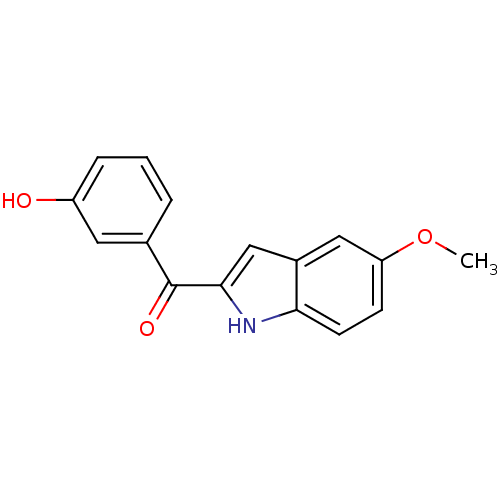 ((3-Hydroxy-phenyl)-(5-methoxy-1H-indol-2-yl)-metha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474300 (CHEMBL116173) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474308 (CHEMBL112385) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM97233 (CHEMBL9514 | MLS001164242 | N-[6-(2-thenoyl)-1H-be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107656 ((3,5-Dimethoxy-phenyl)-(5-methoxy-1H-indol-2-yl)-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107676 ((3,4-Dimethoxy-phenyl)-(5-methoxy-1H-indol-2-yl)-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107664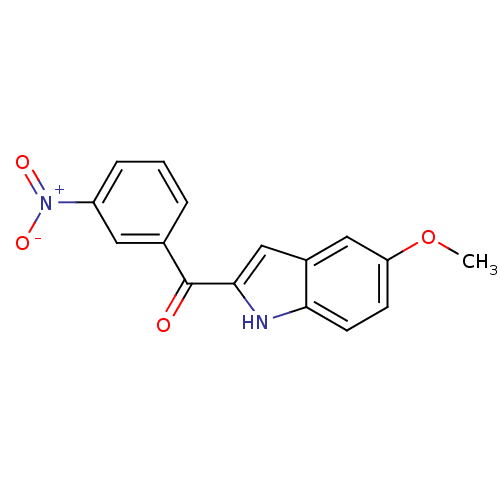 ((5-Methoxy-1H-indol-2-yl)-(3-nitro-phenyl)-methano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107674 (Butyric acid 3-(5-methoxy-1H-indole-2-carbonyl)-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107693 ((5-Methyl-1H-indol-2-yl)-(3,4,5-trimethoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474286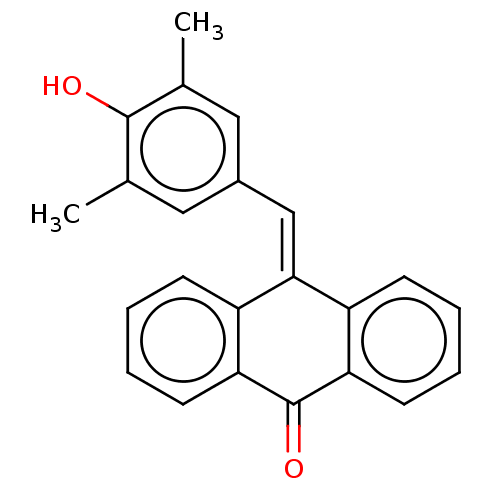 (CHEMBL419387) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474287 (CHEMBL115488) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107651 ((5-Methoxy-1H-indol-2-yl)-(3,4,5-trimethoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107662 ((3-Amino-phenyl)-(5-methoxy-1H-indol-2-yl)-methano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107663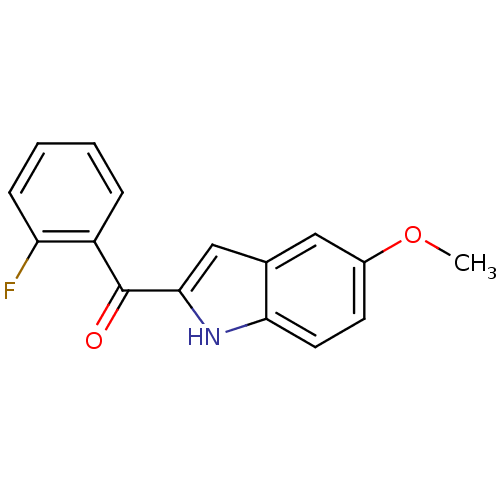 ((2-Fluoro-phenyl)-(5-methoxy-1H-indol-2-yl)-methan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50469124 (CHEMBL113332) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50014846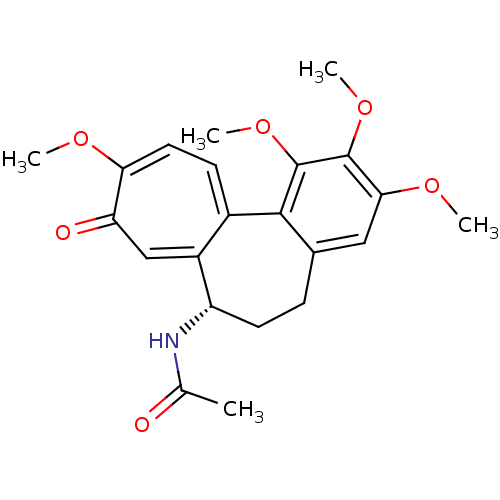 ((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibitory concentration required to displace [3H]colchicine from its binding site on tubulin | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107670 ((5-Methoxy-1H-indol-2-yl)-(2-methoxy-phenyl)-metha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50014846 ((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107671 ((5-Methoxy-1H-indol-2-yl)-(3-trifluoromethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107680 ((5-Methoxy-1H-indol-2-yl)-p-tolyl-methanone | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107682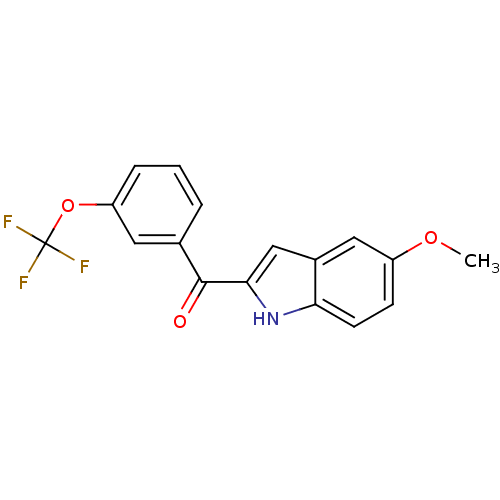 ((5-Methoxy-1H-indol-2-yl)-(3-trifluoromethoxy-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474291 (CHEMBL326388) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474294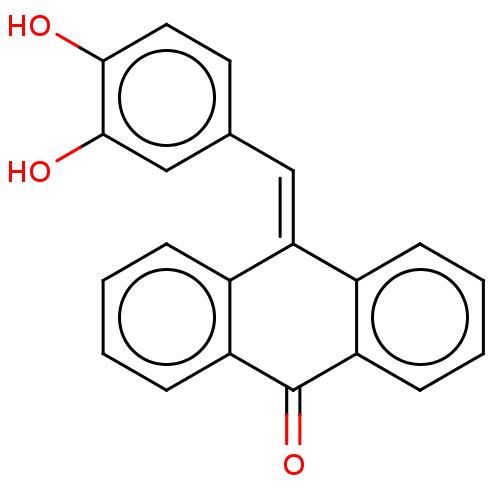 (CHEMBL115360) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107673 ((3-Difluoromethylsulfanyl-phenyl)-(5-methoxy-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474292 (CHEMBL114642) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474307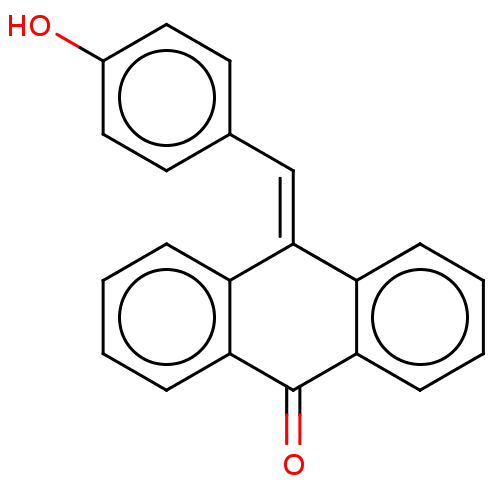 (CHEMBL114651) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107661 ((3,4-Dichloro-phenyl)-(5-methoxy-1H-indol-2-yl)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474306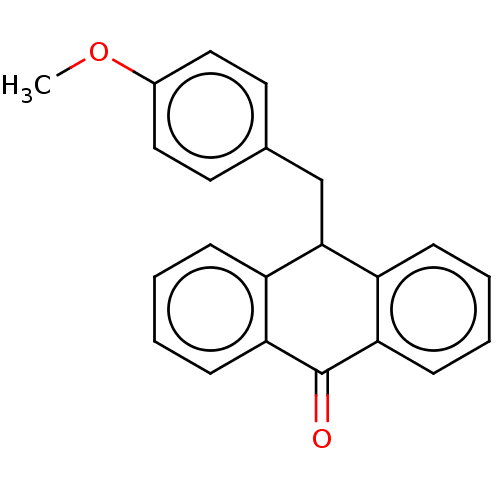 (CHEMBL326114) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107653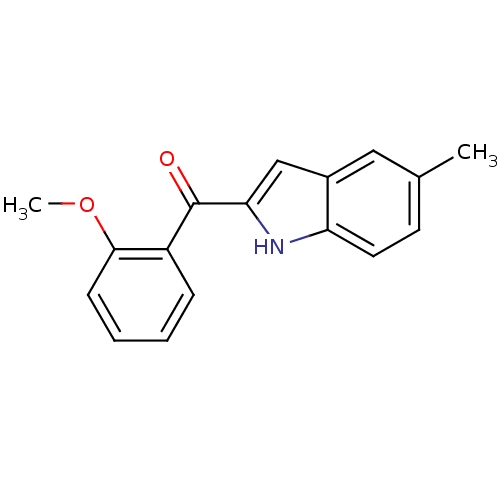 ((2-Methoxy-phenyl)-(5-methyl-1H-indol-2-yl)-methan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107652 ((4-Bromo-phenyl)-(5-methoxy-1H-indol-2-yl)-methano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50474299 (CHEMBL267340) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Westphalian Wilhelms-University Curated by ChEMBL | Assay Description In vitro inhibition of maximum porcine tubulin assembly rate after 20 min at 37 degrees C | J Med Chem 46: 3382-94 (2003) Article DOI: 10.1021/jm0307685 BindingDB Entry DOI: 10.7270/Q2VT1VVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107695 ((5-Methoxy-1H-indol-2-yl)-o-tolyl-methanone | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107654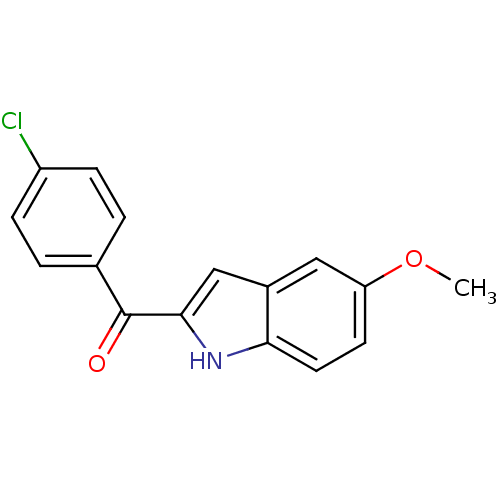 ((4-Chloro-phenyl)-(5-methoxy-1H-indol-2-yl)-methan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-2B chain (Bos taurus) | BDBM50107667 ((2,5-Dimethyl-phenyl)-(5-methoxy-1H-indol-2-yl)-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization. | J Med Chem 44: 4535-53 (2001) BindingDB Entry DOI: 10.7270/Q2BZ65CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |